Question: I need help creating a reaction scheme. FORE COMING TO LAB, IN YOUR LABORATORY NOTEBOOK, CREATE A BRIEF PRE-LAB THAT INCLUDES - a graphic of

I need help creating a reaction scheme.
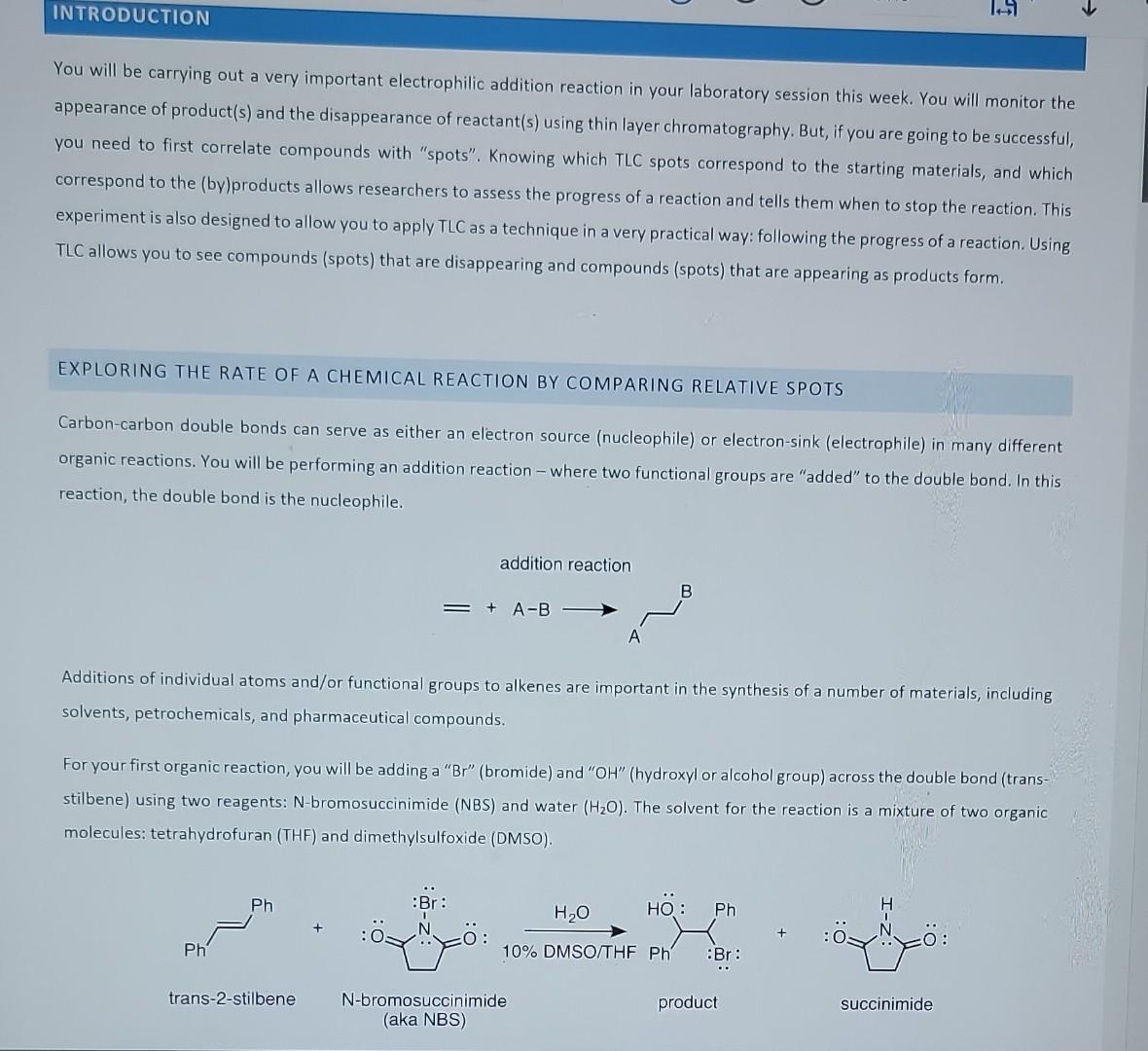
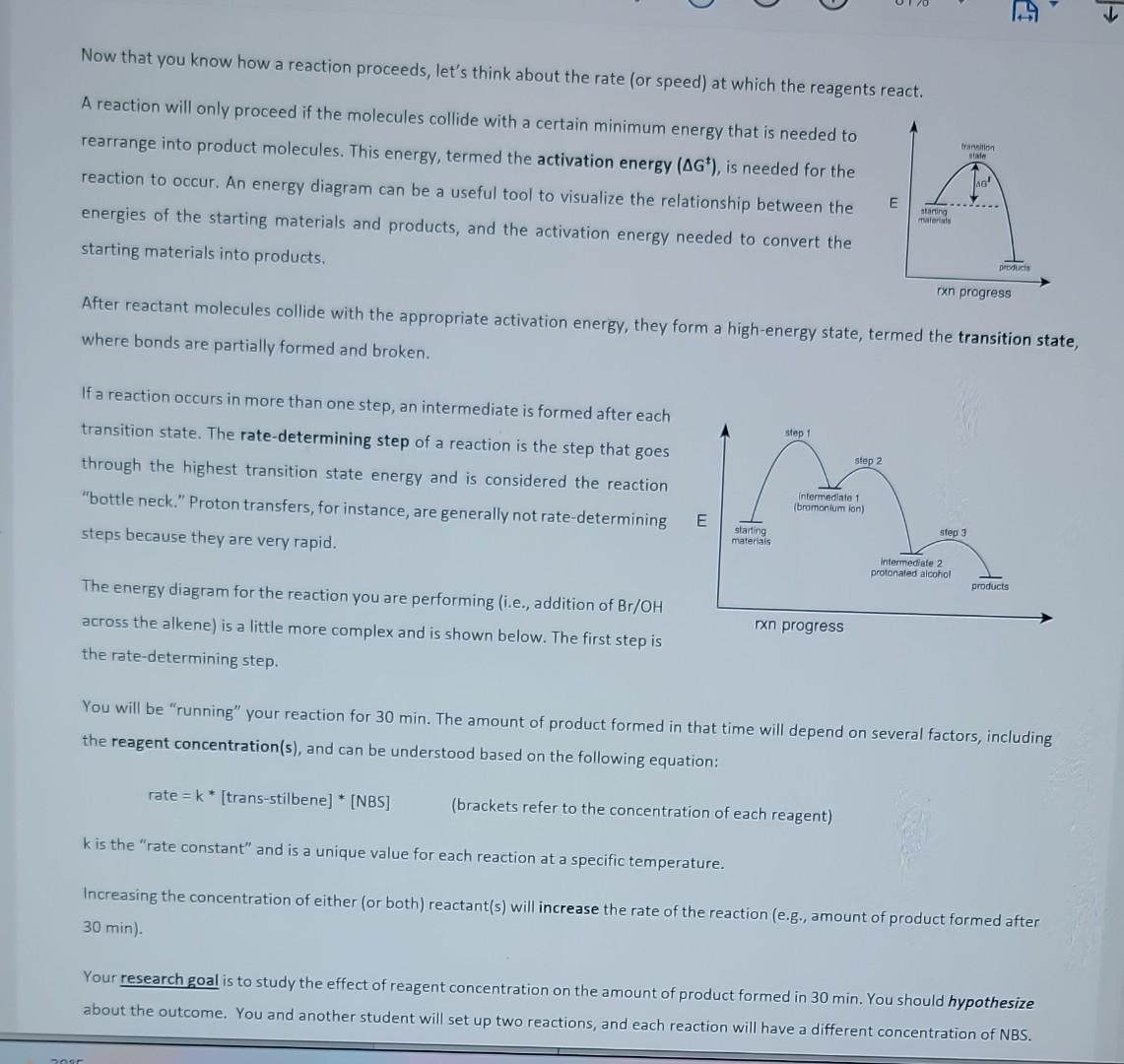
FORE COMING TO LAB, IN YOUR LABORATORY NOTEBOOK, CREATE A BRIEF PRE-LAB THAT INCLUDES - a graphic of your reaction (also known as a reaction scheme), - a hypothesis about what outcome you expect when examining the different conditions Experiment 4: Your First Reaction! - a reagent table (see example below) - an abbreviated procedure (see below, one reaction per person). This is what you will use to carry out the reaction! You will be carrying out a very important electrophilic addition reaction in your laboratory session this week. You will monitor the appearance of product(s) and the disappearance of reactant(s) using thin layer chromatography. But, if you are going to be successful, you need to first correlate compounds with "spots". Knowing which TLC spots correspond to the starting materials, and which correspond to the (by)products allows researchers to assess the progress of a reaction and tells them when to stop the reaction. This experiment is also designed to allow you to apply TLC as a technique in a very practical way: following the progress of a reaction. Using TLC allows you to see compounds (spots) that are disappearing and compounds (spots) that are appearing as products form. EXPLORING THE RATE OF A CHEMICAL REACTION BY COMPARING RELATIVE SPOTS Carbon-carbon double bonds can serve as either an electron source (nucleophile) or electron-sink (electrophile) in many different organic reactions. You will be performing an addition reaction - where two functional groups are "added" to the double bond. In this reaction, the double bond is the nucleophile. Additions of individual atoms and/or functional groups to alkenes are important in the synthesis of a number of materials, including solvents, petrochemicals, and pharmaceutical compounds. For your first organic reaction, you will be adding a "Br" (bromide) and "OH" (hydroxyl or alcohol group) across the double bond (transstilbene) using two reagents: N-bromosuccinimide (NBS) and water (H2O). The solvent for the reaction is a mixture of two organic molecules: tetrahydrofuran (THF) and dimethylsulfoxide (DMSO). After reactant molecules collide with the appropriate activation energy, they form a high-energy state, termed the transition state, where bonds are partially formed and broken. If a reaction occurs in more than one step, an intermediate is formed after each transition state. The rate-determining step of a reaction is the step that goes through the highest transition state energy and is considered the reaction "bottle neck." Proton transfers, for instance, are generally not rate-determining steps because they are very rapid. The energy diagram for the reaction you are performing (i.e., addition of Br/OH across the alkene) is a little more complex and is shown below. The first step is the rate-determining step. You will be "running" your reaction for 30min. The amount of product formed in that time will depend on several factors, including the reagent concentration(s), and can be understood based on the following equation: rate=k[trans-stilbene][[NBS] (brackets refer to the concentration of each reagent) k is the "rate constant" and is a unique value for each reaction at a specific temperature. Increasing the concentration of either (or both) reactant(s) will increase the rate of the reaction (e.g., amount of product formed after 30min). Your research goal is to study the effect of reagent concentration on the amount of product formed in 30 min. You should hypothesize about the outcome. You and another student will set up two reactions, and each reaction will have a different concentration of NBS
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


