Question: Amino acids contain both a basic functional group, the amine, and an acidic functional group, the carboxylic acid. Thus, they can undergo an internal acid-base
Amino acids contain both a basic functional group, the amine, and an acidic functional group, the carboxylic acid. Thus, they can undergo an internal acid-base re-action as shown in the following equation for the amino acid phenylalanine:
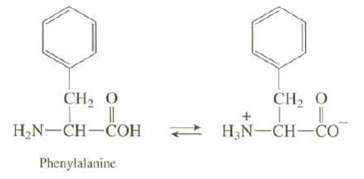
Using Table 4.2 and neglecting the effect of one group on the acidity of the other, predict the position of this equilibrium in the case of phenylalanine. Explain whether your prediction is in accord with the experimental observations that phenylalanine melts at 273-276oC and is very soluble in water.
CH, 0 || CH, 0 T HN-CH-COH
Step by Step Solution
3.38 Rating (173 Votes )
There are 3 Steps involved in it
From Table 42 the p K a for NH 4 is 924 and the p K a for a car... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
15-C-O-A-B-R (41).docx
120 KBs Word File


