Question: Ammonia is oxidized in a well-insulated continuous reactor: 4 NH 3 (g) + 5 O 2 (g) ? 4 NO (g) + 6 H 2
Ammonia is oxidized in a well-insulated continuous reactor: 4 NH3 (g) + 5 O2 (g) ? 4 NO (g) + 6 H2O (v): ?H?? = ? 904.7 kJ/mol
The feed stream enters at 200?C and the products leave at temperature T out?(?C). The inlet?outlet enthalpy table for the reactor appears as follows:
(a) Draw and label a process flowchart and calculate the molar amounts of the product stream components and the extent of reaction, ? (mol/s). Fill in the values of n3, n4, and n5 on the enthalpy table.
(b) The energy balance for this reactor reduces to ?H ? 0. Summarize the assumptions that must be made to obtain this result.
(c) Calculate the values of H1 and H2 and write expressions for H3, H4, and H5 in terms of the outlet temperature. T out Then calculate T out from the energy balance, using either a spreadsheet or a programmable calculator.?
(d) A design engineer obtained a preliminary estimate of the reactor outlet temperature using only the first terms of the heat capacity formulas in Table B.2. [For example, (Cp) NH3 ? 0.03515 kJ / (mol??C).j what value did she calculate? Taking the result of part (c) to be correct, determine the percentage error in T out that results from using the one-term heat capacity formulas.
(e) The preliminary estimate of T01 was mistakenly used as the basis of the design and construction of the reactor. Was this a dangerous error from the standpoint of reactor safety or did it in fact lower the hazard potential? Explain.
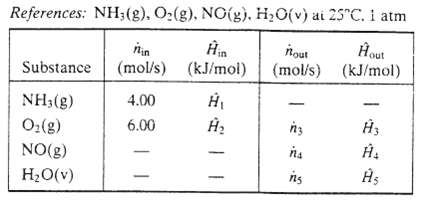
References: NH3(g), O(g), NO(g), HO(v) ai 25C. I atm in out Substance (mol/s) (kJ/mol) (kJ/mol) NH3(g) O(g) NO(g) HO(v) 4.00 6.00 nout (mol/s) (mol/s) n3 na ng 5
Step by Step Solution
3.39 Rating (171 Votes )
There are 3 Steps involved in it
a 4NH3g 50g 4NOg 6HOg AH 9047 kJmol Basis 10 mols Feed gas b C 4 mols NH3 6 mols 0 Tin 200C O consum... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
13-E-C-E-C-P (537).docx
120 KBs Word File


