Question: Problem 2 (13 points). Ammonia is oxidized into nitric oxide and water as shown by the reaction below. 4 NH3 (8) + 5 O2(g) 4
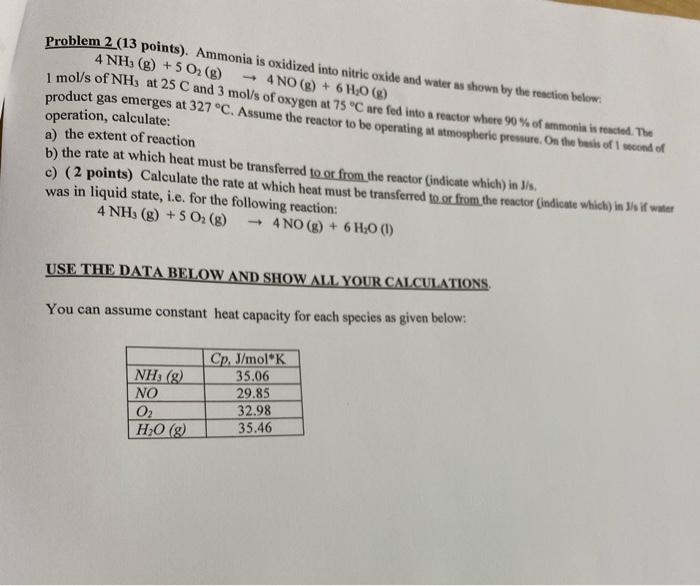
Problem 2 (13 points). Ammonia is oxidized into nitric oxide and water as shown by the reaction below. 4 NH3 (8) + 5 O2(g) 4 NO(g) + 6H20 (g) 1 mol/s of NH3 at 25 C and 3 mol/s of oxygen at 75 C are fed into a reactor where 90% of ammonia is readed. The product gas emerges at 327 C. Assume the reactor to be operating at atmospheric premure. On the basis of 1 second of operation, calculate: a) the extent of reaction b) the rate at which heat must be transferred to or from the reactor (indicate which) in S/s. c) (2 points) Calculate the rate at which heat must be transferred to ot from the reactor (indicate which) in Jis if water was in liquid state, i.e. for the following reaction: 4 NH3(g) + 5 O2 (g) - 4 NO(g) + 6H20 (1) USE THE DATA BELOW AND SHOW ALL YOUR CALCULATIONS, You can assume constant heat capacity for each species as given below: NH; (g) NO 02 HO (8) Cp, J/mol K 35.06 29.85 32.98 35.46 Problem 2 (13 points). Ammonia is oxidized into nitric oxide and water as shown by the reaction below. 4 NH3 (8) + 5 O2(g) 4 NO(g) + 6H20 (g) 1 mol/s of NH3 at 25 C and 3 mol/s of oxygen at 75 C are fed into a reactor where 90% of ammonia is readed. The product gas emerges at 327 C. Assume the reactor to be operating at atmospheric premure. On the basis of 1 second of operation, calculate: a) the extent of reaction b) the rate at which heat must be transferred to or from the reactor (indicate which) in S/s. c) (2 points) Calculate the rate at which heat must be transferred to ot from the reactor (indicate which) in Jis if water was in liquid state, i.e. for the following reaction: 4 NH3(g) + 5 O2 (g) - 4 NO(g) + 6H20 (1) USE THE DATA BELOW AND SHOW ALL YOUR CALCULATIONS, You can assume constant heat capacity for each species as given below: NH; (g) NO 02 HO (8) Cp, J/mol K 35.06 29.85 32.98 35.46
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


