Question: An aqueous process stream of 100 gal/h at 2 o C contains 8 wt% Na 2 SO 4 and 6 wt% of a high-molecular-weight substance
An aqueous process stream of 100 gal/h at 2oC contains 8 wt% Na2SO4 and 6 wt% of a high-molecular-weight substance (A). This stream is processed in a continuous, counter current-flow dialyzer using a pure water sweep of the same flow rate. The membrane is a microporous cellophane with pore volume = 50%, wet thickness = 0.0051 cm, tortuosity = 4.1, and pore diameter = 31A. The molecules to be separated have the following properties:
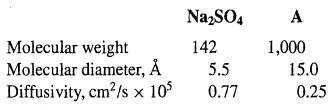
Calculate the membrane area in m2 for only a 10% transfer of A through the membrane, assuming no transfer of water. What is the percent recovery of the Na2SO4 in the diffusate? Use log-mean concentration driving forces and assume that the mass-transfer resistances on each side of the membrane are each 25% of the total mass-transfer resistances for Na2SO4 andA.
NazSO4 A Molecular weight Molecular diameter, 142 5.5 1,000 15.0 0.25 Diffusivity, cm/s x 105 0.77
Step by Step Solution
3.42 Rating (161 Votes )
There are 3 Steps involved in it
Subject Dialysis of an aqueous stream to separate Na2SO4 from a highmolecularweight substance A Given Aqueous feed of 100 galh at 20oC containing 8 wt ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
37-E-C-E-S-P (499).docx
120 KBs Word File


