Question: An innovative engineer has invented a device to replace the hydraulic jacks found at many service stations. A movable piston with a diameter of 0.15
An innovative engineer has invented a device to replace the hydraulic jacks found at many service stations. A movable piston with a diameter of 0.15 m is fitted into a cylinder. Cars are raised by opening a small door near the base of the cylinder, inserting a block of dry ice (solid CO2), closing and sealing the door, and vaporizing the dry ice by applying just enough heat to raise the cylinder contents to ambient temperature (25?C). The car is subsequently lowered by opening a valve and venting the cylinder gas.
The device is tested by raising a car a vertical distance of 1.5 m. The combined mass of the piston and the car is 5500 kg. Before the piston rises, the cylinder contains 0.030 m3 of CO2 at ambient temperature and pressure (1atm). Neglect the volume of the dry ice.
(a) Calculate the pressure in the cylinder when the piston comes to rest at the desired elevation.
(b) How much dry ice (kg) must be placed in the cylinder? Use the SRK equation of state for this calculation.
(c) Outline how you would calculate the minimum piston diameter required for any elevation of the car to occur if the calculated amount of dry ice is added. (Just give formulas and describe the procedure you would follow?no numerical calculations required)
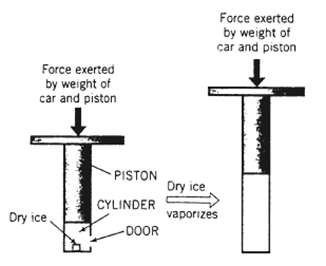
Force exerted by weight of car and piston Dry ice. PISTON CYLINDER -DOOR Force exerted by weight of car and piston Dry ice vaporizes
Step by Step Solution
3.56 Rating (167 Votes )
There are 3 Steps involved in it
a Pco W Pco A W A mco F Pco AW0 where Wmg5500 kg981 53900 N 301 atm piston b SRK equa... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (2 attachments)
13-E-C-E-C-P (230).pdf
180 KBs PDF File
13-E-C-E-C-P (230).docx
120 KBs Word File


