Question: Aromatic compounds such as benzene react with alkyl chlorides in the presence of A1C1 3 catalyst to yield alkylbenzenes. The reaction occurs through a carbocation
Aromatic compounds such as benzene react with alkyl chlorides in the presence of A1C13 catalyst to yield alkylbenzenes. The reaction occurs through a carbocation intermediate, formed by reaction of the alkyl chloride with A1C13 (R-C1 + AlCl3 ? R+ + A1C14-). How can you explain the observation that reaction of benzene with 1-chioropropane yields isopropylbenzene as the major product?
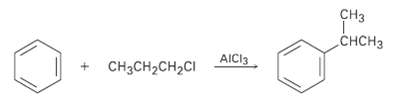
CHCH3 AICI3 CCH-CH2CI
Step by Step Solution
3.41 Rating (164 Votes )
There are 3 Steps involved in it
Reaction of 1chloropropane with the Lewis acid AlCl3 forms a ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
22-C-OC-A (134).docx
120 KBs Word File


