At a pressure of 490 kPa, the saturation temperature of sulfur dioxide (SO2) is 32?C, the density
Question:
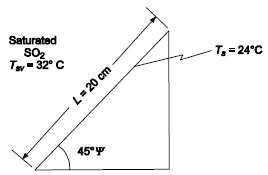
At a pressure of 490 kPa, the saturation temperature of sulfur dioxide (SO2) is 32?C, the density is 1350 kg/m3, the heat of vaporization is 343 kJ/kg, the absolute viscosity is 3.2 x 10??4 (Ns)/m2, the specific heat is 1445 J/(kg K) and the thermal conductivity is 0.192 W/(m K). If the SO2 is to be condensed at 490 kPa on a 20??cm flat surface, inclined at an angle at 45?, whose temperature is maintained uniformly at 24?C, calculate(a) The thickness of the condensate film 1.3 cm from the bottom,(b) The average heat transfer coefficient for the entire plate, and(c) The rate of condensation in kilograms per hour.GIVENSO2 condensing on a flat surface inclined 45?Pressure (p) = 490 kPaSO2 propertiesSaturation temperature (Tsv) = 32?CLiquid density (ρl) = 1350 kg/m3Heat of vaporization (hfg) = 343 kJ/kg = 343, 000 J/kgAbsolute viscosity (μl) = 3.2 x 10??4 (Ns)/m2Specific heat (cpl = 1445 J/(kg K))Thermal conductivity (k) = 0.192 W/(m K)Surface temperature (Ts) = 24?C (uniform)Length of inclined edge of surface (L) = 20 cm = 0.2 mASSUMPTIONSSteady stateLaminar condensate flowVapor density is negligible compared to the liquid densityInterfacial shear and momentum effects arenegligible
Step by Step Answer:

Principles of heat transfer
ISBN: 978-0495667704
7th Edition
Authors: Frank Kreith, Raj M. Manglik, Mark S. Bohn





