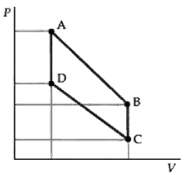
Question: At point D in Figure the pressure and temperature of 2 mol of an ideal monatomic gas are 2 atm and 360 K. The volume
At point D in Figure the pressure and temperature of 2 mol of an ideal monatomic gas are 2 atm and 360 K. The volume of the gas at point B on the PV diagram is three times that at point D and its pressure is twice that at point C. Paths AB and CD represent isothermal processes. The gas is carried through a complete cycle along the path DABCD. Determine the total amount of work done by the gas and the heat supplied to the gas along each portion of thecycle.
B.
Step by Step Solution
3.32 Rating (167 Votes )
There are 3 Steps involved in it
The volume at D from V nRT P V D 2 8314 360202 L 296 L We f... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
10-P-T-F-L (546).docx
120 KBs Word File


