Benzene can be used to break the ethanol/water azeotrope so as to produce nearly pure ethanol. The
Question:
Benzene can be used to break the ethanol/water azeotrope so as to produce nearly pure ethanol. The Wilson constants for the ethanol(l)/benzene(2) system at 45oC are A12 = 0.124 and A21 = 0.523. Use these constants with the Wilson equation to predict the liquid-phase activity coefficients for this system over the entire range of composition and compare them, in a plot likeFigure, with the following experimentalresults.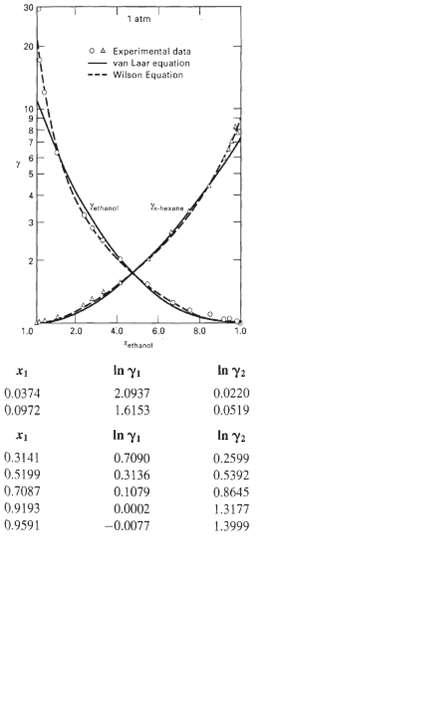
Transcribed Image Text:
30 1 atm 20 O A Experimental data van Laar equation Wilson Equation 10 Yethano Yheane 2.0 4.0 6.0 8.0 1.0 1.0 Tethanal In y: In yi 2.0937 0.0220 0.0374 0.0972 1.6153 0.0519 In Yi In y2 0.2599 0.5392 0.3141 0.5199 0.7090 0.3136 0.7087 0.9193 0.1079 0.8645 0.0002 1.3177 0.9591 -0.0077 1.3999
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (8 reviews)
Let 1 ethanol and 2 benzene The Wilson constants are L12 0124 an...View the full answer

Answered By

Diane Joyce Pastorin
Please accept my enthusiastic application to solutioninn. I would love the opportunity to be a hardworking, passionate member of your tutoring program. As soon as I read the description of the program, I knew I was a well-qualified candidate for the position.
I have extensive tutoring experience in a variety of fields. I have tutored in English as well as Calculus. I have helped students learn to analyze literature, write essays, understand historical events, and graph parabolas. Your program requires that tutors be able to assist students in multiple subjects, and my experience would allow me to do just that.
You also state in your job posting that you require tutors that can work with students of all ages. As a summer camp counselor, I have experience working with preschool and kindergarten-age students. I have also tutored middle school students in reading, as well as college and high school students. Through these tutoring and counseling positions, I have learned how to best teach each age group.
4.60+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Chemical Engineering questions
-
The basic barometer can be used to measure the height of a building. If the barometric readings at the top and at the bottom of a building are 730 and 755 mm Hg, respectively, determine the height of...
-
A laser beam can be used to weld, drill, etch, cut. And mark metals, as shown In Figure P5.3(a) [14]. Assume we have a work requirement for an accurate laser to mark a parabolic path with a...
-
A deburring robot can be used to smooth off machined parts by following a preplanned path (input command signal). In practice, errors occur due to robot inaccuracy, machining errors, large...
-
Children out of School in America is a report on delinquency of school-age children prepared by the Childrens Defense Fund (CDF), a government-sponsored organization. Consider the following three...
-
Indicate how (a) Trading investments, (b) Investments in associates, (c) Debt investments held to maturity are classified on the statement of financial position.
-
Using the grammar in Example 3.4, show a parse tree and a leftmost derivation for each of the following statements: A = (A + B) * C Data From Example 3.4: A | B|C + | * | ( ) |
-
Should medical advice be dispensed on the telephone? Explain your opinion.
-
Lindstrom Design Services billed its customers a total of $245,100 for the month of August, including 9 percent federal excise tax and 5 percent sales tax. 1. Determine the proper amount of service...
-
Sunland Manufacturing uses a job order cost system. On April 1, the company has Work in Process Inventory of $7,130 and two jobs in process: Job No. 221, $3,370, and Job No. 222, $3,760. During...
-
A piece of string is connected between two adjacent houses to form a clothesline. The houses are separated by d = 22 m, and both ends of the string are the same height off the ground. A sweater is...
-
The sharp separation of benzene and cyclohexane by distillation at ambient pressure is impossible because of the formation of an azeotrope at 77.6oC. K.C. Chao [Ph.D. thesis, University of Wisconsin...
-
For the binary system ethanol(l)/isooctane(2) at 50?C, the infinite-dilution, liquid-phase activity coefficients are ?1? = 21.17 and ?2? = 9.84.(a) Calculate the constants A12 and All in the van Laar...
-
Suppose the U.S. government defaults on its payments (i.e., cannot pay T-bills at their maturity date). What would be the effect on the T-bill rate? What would be the effect on the interest rates of...
-
True Or False In no-fault states, the right to sue for torts is retained for intentional injuries inflicted with an automobile and injuries caused by intoxicated drivers.
-
In a no-fault state a. an injured party cannot sue for intentional injuries inflicted with an automobile. b. an injured party cannot sue for injuries caused by an intoxicated driver. c. thresholds...
-
What might an insured do to protect themself from an excess judgment?
-
Under the reasonable expectations doctrine, a. the court expects both parties to be reasonable. b. the court will reform the contract to meet the reasonable expectation of the nondrafter of the...
-
__________ insurance is where insureds carrier would provide coverage to insured for bodily injury or property damage while operating a vehicle.
-
An analysis of costs of Sullivan Manufacturing Company gives the following information. You are required to determine (a) Break-even sales volume (b) Profit at the budgeted sales of 18,50,000. Cost...
-
For the vector whose polar components are (Vr = 1, Vθ = 0), compute in polars all components of the second covariant derivative Vα;μ;ν. To find...
-
Show that if x ~ Fv i ,v 2 then VIX V + Vx ~ Be (v, 1).
-
An aqueous acetic acid solution containing 6.0 mol/L of acid is extracted with chloroform at 25 C to recover the acid (B) from chloroform- insoluble impurities in the water. The water (A) and...
-
What is a group method of calculation?
-
(a) When rinsing clothes, would it be more efficient to divide the water and rinse several times, or should one use all the water in one rinse? Explain. (b) Devise a washing machine that gives the...
-
How should an organization deal with third-party network access?
-
a) In a circuit under test a resistor, R, is connected in series with a capacitor, C. An a.c. current, i, flows through this RC combination, causing a voltage (VR) of 6V to be developed across the...
-
Assignment Instructions: Complete your Capstone Project paper by submitting a project based on previous research and coursework that you have completed in Cybersecurity or on a Cybersecurity topic...

Study smarter with the SolutionInn App


