Question: For the binary system ethanol(l)/isooctane(2) at 50?C, the infinite-dilution, liquid-phase activity coefficients are ?1? = 21.17 and ?2? = 9.84.(a) Calculate the constants A12 and
For the binary system ethanol(l)/isooctane(2) at 50?C, the infinite-dilution, liquid-phase activity coefficients are ?1? = 21.17 and ?2? = 9.84.(a) Calculate the constants A12 and All in the van Laar equations.(b) Calculate the constants A12 and A21 in the Wilson equations.(c) Using the constants from (a) and (b), calculate ?1 and ?2 over the entire composition range and plot the calculated points as log ? versus x1.(d) How well do the van Laar and Wilson predictions agree with the azeotropic point where xl = 0.5941, ?l = 1.44, and ?2 = 2.18?(e) Show that the van Laar equation erroneously predicts separation into two liquid phases over a portion of the composition range by calculating and plotting a y-x diagram like Figure.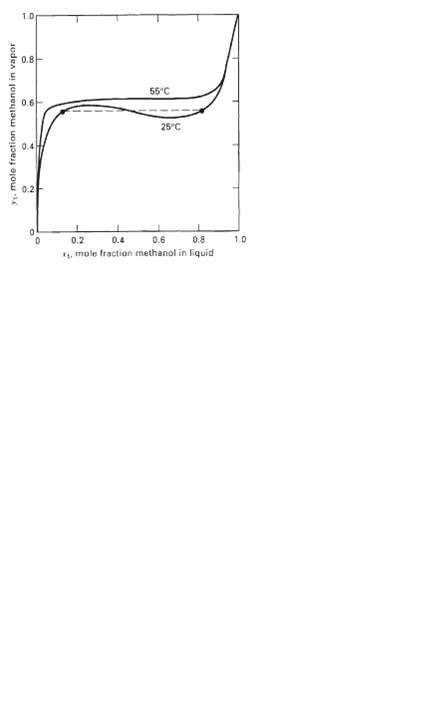
0.8 55C 0.6 25C 0.2 0.4 0.6 0.8 1.0 0.2 4, mole fraction methanol in Fquid y. mole fraction methanol in vapor
Step by Step Solution
3.44 Rating (163 Votes )
There are 3 Steps involved in it
a From van Laar Eqs 272 for infinite dilution b From Wilson Eqs 280 and 281 for infinite dilution So... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
37-E-C-E-S-P (49).docx
120 KBs Word File


