Question: Benzene has an ultraviolet absorption at ? max = 204mm, and para-toluidine has ? max = 235nm. How do you account for this difference? -NH2
Benzene has an ultraviolet absorption at ? max = 204mm, and para-toluidine has ? max = 235nm. How do you account for this difference?
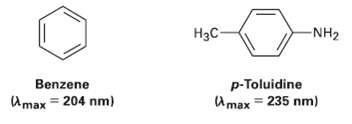
-NH2 p-Toluidine U max = 235 nm) Benzene Amax = 204 nm)
Step by Step Solution
3.25 Rating (163 Votes )
There are 3 Steps involved in it
Double bonds can be conjugated not only with other multiple bonds but also w... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
22-C-O-S (299).docx
120 KBs Word File


