Question: Compound c is a slightly stronger acid than compound d. The CH3O group has both an inductive effect and a resonance effect on the acidity
Compound c is a slightly stronger acid than compound d. The CH3O group has both an inductive effect and a resonance effect on the acidity of d.
(a) Explain how the inductive effect of the CH3O group should affect the acidity of d.
(b) Show the resonance structures for d that involve the CH3O group. Would you expect the resonance effect of the CH3O group to cause an increase or decrease in acidity?
(c) In situations like this, the resonance effect is usually larger than the inductive effect. Is this consistent with the experimental acidities of these twocompounds?
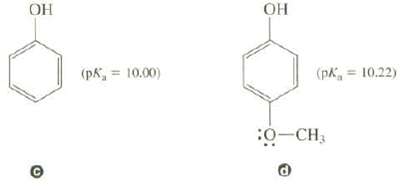
(PR = 10.00) OH (pK = 10.22) 30-CH3
Step by Step Solution
3.42 Rating (171 Votes )
There are 3 Steps involved in it
a T he CH 3 O group has an electronwithdrawing induct... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
15-C-O-A-B-R (47).docx
120 KBs Word File


