Question: Consider the titration in Figure 15-2. (a) Write a balanced titration reaction. (b) Write two different half-reactions for the indicator electrode. (c) Write two different
Consider the titration in Figure 15-2.
(a) Write a balanced titration reaction.
(b) Write two different half-reactions for the indicator electrode.
(c) Write two different Nernst equations for the cell voltage.
(d) Calculate E at the following volumes of Ce4+: 10.0, 25.0, 49.0, 50.0, 51.0, 60.0, and 100.0 mL. Compare your results with Figure 15-2.
Figure 15-2
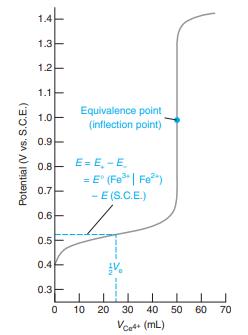
1.4 1.3 1.2 1.1 Equivalence point (inflection point) 1.0 0.9 0.8- E= E, -E = E" (Fe| Fe") - E (S.C.E.) 0.7 0.6- 0.5 0.4 0.3 10 20 30 40 50 60 70 Vcet+ (mL) Potential (V vs. S.C.E.)
Step by Step Solution
3.37 Rating (166 Votes )
There are 3 Steps involved in it
a b c d Ce4 Fe2 Ce Fe Fe3 efe2 Eo 0767 V Ce4eCe3 E 170 V E 07670059 16 log 100 mL E ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
878-E-C-E-E-C (2079).docx
120 KBs Word File


