Question: Data below come from the graph in Box 14-2, for which the separate solutions method was used to measure selectivity coefficients for a sodium ion-selective
Data below come from the graph in Box 14-2, for which the separate solutions method was used to measure selectivity coefficients for a sodium ion-selective electrode at 21.5°C. Use Equation 14-11 to calculate log KPot for each line below.
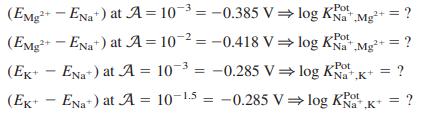
Equation 14-11
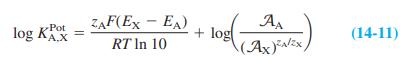
(EMg+ - ENa) at A= 103 = -0.385 V = log Kot. Mg 2+ = ? Na" Mg (EMg+ - ENa") at A= 10 = -0.418 V = log K Mg+ = ? Pot Pot (Ex+ - ENa) at A = 10 = -0.285 V = log KK = (EK+ - ENa) at A = 10-1.5 = -0.285 V log Kt Pot ,K* = ? %3D
Step by Step Solution
3.40 Rating (169 Votes )
There are 3 Steps involved in it
For the first line of data with A Nat and X Mg The fi... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
878-E-C-E-E-C (2067).docx
120 KBs Word File


