Deposition of silver from a turbulent stream (Fig. 21B.3), an approximately 0.1 N solution of KNO3 containing
Question:
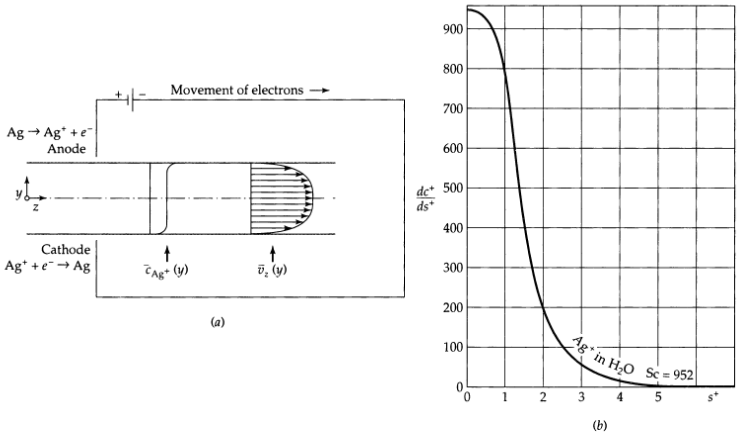
Deposition of silver from a turbulent stream (Fig. 21B.3), an approximately 0.1 N solution of KNO3 containing 1.00 x 10-6 g-equiv. AgNO3 per liter is flowing between parallel Ag plates, as shown in Fig. 21B.3 (a). A small voltage is applied across the plates to produce a deposition of Ag on the cathode (lower plate) and to polarize the circuit completely (that is, to maintain the Ag+ concentration at the cathode very nearly zero). Forced diffusion may be ignored, and the Ag+ may be considered to be moving to the cathode by ordinary (that is, Fickian) diffusion and eddy diffusion only. Furthermore, this solution is sufficiently dilute that the effects of the other ionic species on the diffusion of Ag+ are negligible.
(a) Calculate the Ag+ concentration profile, assuming that (i) the effective binary diffusivity of Ag+ through water is 1.06 x 10-5 cm2/s; (ii) the truncated Lin, Moulton, and Putnam expression of Eq. 5.4-2 for the turbulent velocity distribution in round tubes is valid for "slit flow" as well, if four times the hydraulic radius is substituted for the tube diameter; (iii) the plates are 1.27 cm apart, and √τ0/p is 11.4 cm/s.
(b) Estimate the rate of deposition of Ag on the cathode, neglecting all other electrode reactions.
(c) Does the method of calculation in part (a) predict a discontinuous slope for the concentration profile at the center plane of the system? Explain.
The word "distribution" has several meanings in the financial world, most of them pertaining to the payment of assets from a fund, account, or individual security to an investor or beneficiary. Retirement account distributions are among the most...
Step by Step Answer:






