Diamond, an allotrope of carbon, is the hardest substance and the best conductor of heat yet characterized.
Question:
Diamond, an allotrope of carbon, is the hardest substance and the best conductor of heat yet characterized. For these reasons, diamond is used widely in industrial applications that require a strong abrasive. Unfortunately, it is difficult to synthesize diamond from the more readily available allotropes of carbon, such as graphite. To illustrate this point,
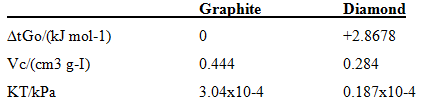
calculate the pressure required to convert graphite into diamond at 25°C. The following data apply to 25°C and 100 kPa. Assume the specific volume; Vs' and KT are constant with respect to pressure changes.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





