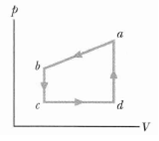
Question: Figure displays a closed cycle for a gas (the figure is not drawn to scale). The change in the internal energy of the gas as
Figure displays a closed cycle for a gas (the figure is not drawn to scale). The change in the internal energy of the gas as it moves from a to c along the path abc is €“ 200 J. As it moves from c to d, 180 J must be transferred to it as heat. An additional transfer of 80 J to it as heat is needed as it moves from d to a. How much work is done on the gas as it moves from c to d?
Step by Step Solution
3.22 Rating (160 Votes )
There are 3 Steps involved in it
We note that there is no work done in the process going from d to a so Qda A... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
2-P-T-F-L (440).docx
120 KBs Word File


