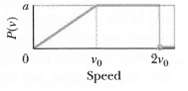
Question: Figure shows a hypothetical speed distribution for a sample of N gas particles (not that P(v) = 0 for speed v > 2v0). What are
Figure shows a hypothetical speed distribution for a sample of N gas particles (not that P(v) = 0 for speed v > 2v0). What are the values of?
(a) Av0,
(b) vavg/v0, and
(c) vrms/v0?
(d) What fraction of the particles has a speed between 1.5v0 and2.0v0?
2vo Speed (A)d
Step by Step Solution
3.46 Rating (169 Votes )
There are 3 Steps involved in it
a The distribution function gives the fraction of particles with speeds between vand v dv so its int... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
2-P-T-K-T (257).docx
120 KBs Word File


