Question: Figure shows two paths that may be taken by a gas from an initial point i to a final point f. Path 1 consists of
Figure shows two paths that may be taken by a gas from an initial point i to a final point f. Path 1 consists of an isothermal expansion (work is 50 J in magnitude), an adiabatic expansion (work is 40 J in magnitude), an isothermal compression (work is 30 J in magnitude), and then an adiabatic compression (work is 25 J in magnitude). What is the change in the internal energy of the gas if the gas goes from point I to point f along path2?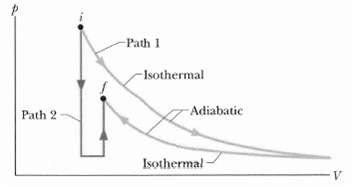
Path 1 -Isothermal -Adiabatic Path 2 Isothermal
Step by Step Solution
3.25 Rating (177 Votes )
There are 3 Steps involved in it
Since AEint does not depend on the type of process Also since for an ideal gas ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
2-P-T-K-T (273).docx
120 KBs Word File


