Question: A flowchart of a methanol synthesis process is shown below. A. Fresh feed?a mixture of CO. H 2 , N 2 , and CO 2
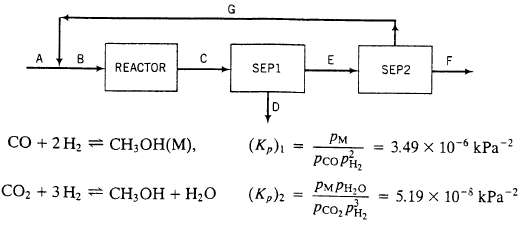
A flowchart of a methanol synthesis process is shown below.
A. Fresh feed?a mixture of CO. H2, N2, and CO2
B. Feed to the reactor?30.O mole% CO, 63.0% H2, 2.0% N2, and 5.0% CO2.
Reactor Two reactions occur and proceed to equilibrium at 200?C and 4925 kPa absolute: The following specifications apply to the labeled streams and process units:
C. Reactor effluent?contains all teed and product species at the reactor temperature and pressure. Species partial pressures satisfy the two given equations.
Sep 1. Condenses all methanol and water in reactor effluent
D. Liquid methanol and water. (These species will be separated by distillation in a unit not shown.)
E. Gas containing N2 and un-reacted CO. H2, and CO2.
Sep2. Multiple-unit separation process
F. All of the nitrogen and some of the hydrogen in Stream E.
G. Recycle stream?CO, CO2 and 10% of the hydrogen fed to Sep2.
(a) Taking 100kmol/h of Stream B as a basis of calculation, calculate the molar flow rates (k mol/h) and molar compositions of the remaining six labeled streams.
(b) The process is to be used to provide 237 k mol/h of methanol. Scale up the flowchart of part (a) to calculate the required fresh feed rate (SCMH), the flow rate of the reactor effluent (SCMH), and the actual volumetric flow rate of the reactor effluent (m3/h), assuming ideal gas behavior.
(c) Use the rule of thumb for a diatomic gas given on p. 192 to test the ideal gas assumption at the reactor outlet. If the assumption is invalid, which of the values calculated in part (b) are in error?
A B REACTOR CO + 2H CHOH(M), C CO + 3H CH3OH + HO SEP1 D (Kp) (Kp)2 = = E PM PPH PMPHO p PH SEP2 -2 = 3.49 x 106 kPa 2 = 5.19 x 108 kPa-
Step by Step Solution
3.25 Rating (166 Votes )
There are 3 Steps involved in it
n kmol CO h n kmol H h n kmol CO h 2 kmol N h kmol CO h i kmol H h 010n kmol H h 0300 kmol COkmol 06... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (2 attachments)
13-E-C-E-C-P (223).pdf
180 KBs PDF File
13-E-C-E-C-P (223).docx
120 KBs Word File


