From the natural abundance of 79 Br and 81 Br, predict the relative amounts of CH 79
Question:
From the natural abundance of 79Br and 81Br, predict the relative amounts of CH79Br3, CH79Br2 81Br, CH79Br81Br2, and CH81Br3. As in Exercise 21-C, the fraction of each isotopic molecule comes from the expansion of (a = b)3, where a is the abun - dance of 79Br and b is the abundance of 81Br Note that (a + b)n =
Compare your answer with Figure 21-7
Figure 21-7
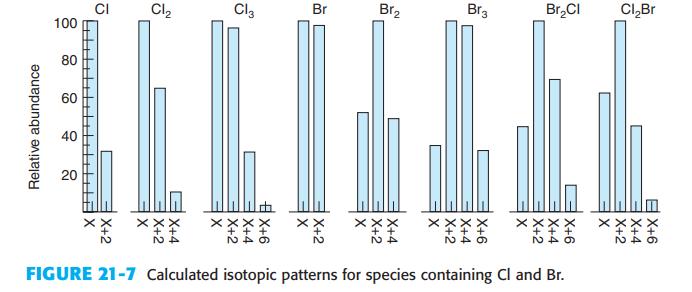
Exercise 21-C
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





