Question: A gas-phase decomposition reaction with stoichiometry 2A ? 2B + C follows a second-order rate law (see Problem 11.14): rd [mo1/(m3?s)] = kC2A where CA
A gas-phase decomposition reaction with stoichiometry 2A ? 2B + C follows a second-order rate law (see Problem 11.14): rd [mo1/(m3?s)] = kC2A where CA is the reactant concentration in moUrn3. The rate constant k varies with the reaction temperature according to the Arrhenius law k[m3/(mol ? s)] = k0 exp(??? E/RT) where k0[m3/(mol ?s]) = the pre-exponential factor E(J/mol = the reaction activation energy R = the gas constant T (K) = the reaction temperature
(a) Suppose the reaction is carried out in a batch reactor of constant volume V (m3) at a constant temperature T (K), beginning with pure A at a concentration CA0. Write a differential balance on A and integrate it to obtain an expression for CA (t) in terms of CA0 and k.
(b) Let P0 (atm) be the initial reactor pressure. Prove that t1/2, the time required to achieve a 50% conversion of A in the reactor, equals RT/kP0, and derive an expression for P1/2, the reactor pressure at this point, in terms of P0. Assume ideal gas behavior.
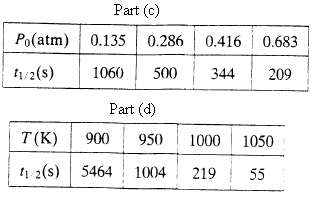
(c) The decomposition of nitrous oxide (N2O) to nitrogen and oxygen is carried out in a 5.00-liter batch reactor at a constant temperature of 1015 K. beginning with pure N2O at several initial pressures. The reactor pressure P(t) is monitored, and the times (t1/2) required to achieve 50% conversion of N2O are noted.
Use these results to verify that the N2O decomposition reaction is second-order and determine the value of k at T = 1015 K.
(d) The same experiment is performed at several other temperatures at a single initial pressure of 1.00 atm, with the following results:
Use a graphical method to determine the Arrhenius law parameters (k0 and E) [or the reaction.
(e) Suppose the reaction is carried out in a batch reactor at T 980 K. beginning with a mixture at 1.20 atm containing 70 mole% N2O and the balance a chemically inert gas. How long (minutes) will it take to achieve a 90% conversion of N2O?
Po(atm) 11/2 (s) Part (c) 0.135 0.286 0.416 0.683 1060 500 344 209 Part (d) 900 950 1000 1050 219 55 T(K) 12(S) 5464 1004
Step by Step Solution
3.46 Rating (159 Votes )
There are 3 Steps involved in it
2A 2BC a Mole balance on A dCV kCV dt t 0 CC40 dC A 1 f 2R fkdt kdt kt CA kt JC A0 C40 Accumulationc... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
13-E-C-E-C-P (600).docx
120 KBs Word File


