Question: A gas sample undergoes a reversible isothermal expansion. Figure gives the change S in entropy of the gas versus the final volume Vf of the
A gas sample undergoes a reversible isothermal expansion. Figure gives the change ΔS in entropy of the gas versus the final volume Vf of the gas. The scale of the vertical axis is set by ΔSs = 64 J/K. How many moles are in the sample?
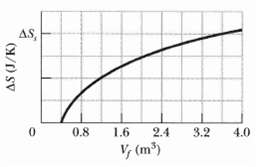
AS, 0.8 1.6 2.4 3.2 4.0 V, (m)
Step by Step Solution
3.31 Rating (157 Votes )
There are 3 Steps involved in it
We concentrate on the first term of Eq 204 the second term is zero because the f... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
2-P-T-S-L (213).docx
120 KBs Word File


