Question: A horizontal cylinder equipped with a friction less piston contains 785 cm 3 of steam at 300 K and 125 kPa. A total of 83.8
A horizontal cylinder equipped with a friction less piston contains 785 cm3 of steam at 300 K and 125 kPa. A total of 83.8 joules of heat is transferred to the steam, causing the steam temperature to rise and the cylinder volume to increase. A constant restraining force is maintained on the piston throughout the expansion, so that the pressure exerted by the piston on the steam remains constant at 125 kPa. The specific enthalpy of steam at 125 kPa varies with temperature approximately as
H (J/mol) = 34,980 + 35.5T (K)
(a) Taking the steam as the system, convince yourself that Q = ΔH for this process—that is. the four conditions specified in part (a) of Problem 7.15 are applicable. Then prove that the final steam temperature is 480 K. Finally, calculate (i) the final cylinder volume (ii) the expansion work done by the steam, and (iii) ΔU(J).
(b) Which of the specified conditions of Problem 7.15 would have been only an approximation if the cylinder were not horizontal?
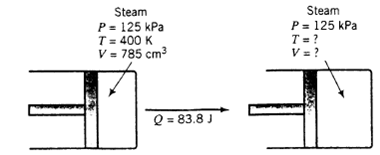
Steam P= 125 kPa T = 400 K V = 785 cm3 Steam P = 125 kPa T = ? V =? Q = 83.8 J
Step by Step Solution
3.44 Rating (170 Votes )
There are 3 Steps involved in it
a AE 0 U 0 AEp 0 no elevation change AP 0 the pressure i... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
13-E-C-E-C-P (369).docx
120 KBs Word File


