Question: You are performing an experiment to measure the specific internal energy of a gas relative to a reference state of 25?C and 1 atm (at
You are performing an experiment to measure the specific internal energy of a gas relative to a reference state of 25?C and 1 atm (at which conditions U is arbitrarily set equal to 0). The gas is placed in a closed insulated 2.10-liter container at 25?C and 1 atm. A switch is alternately closed and opened, causing a current to flow intermittently through an electrical heating coil in the chamber. The gas temperature, which is monitored with a calibrated thermocouple, increases while the circuit is closed and remains constant while it is open. A wattmeter reads 1.4 W when the circuit is closed; 90% of this power is transferred to the gas as heat. The thermocouple calibration curve is a straight line through the points (T = 0?C, E = ?0.249mV) and (T = 100?C, E = 5.27 mV), where E is the thermocouple potentiometer reading. The following data are taken, where t represents the cumulative time during which the circuit was closed:
(a) Which given item of information suggests that the chamber may be considered adiabatic? (Note:
Simply saying the container is insulated does not guarantee that it is adiabatic.)
(b) Write the energy balance for the gas in the chamber and use it to calculate U (J/mol) at each of the observed temperatures, neglecting the work done on the gas by the stirrer. Express your solution as a table of U versus T.
(c) What might the purpose of the stirrer be?
(d) What happens to the 0.14 W of power that does not go to raise the temperature of the gas?
(e) A colleague points out to you that the calculated values of U fail to take something into account and so do not precisely correspond to the values at the calculated temperatures and 1 atm. You reply that she is quite correct, but it does not matter. Justify her statement and state the basis of your reply. Suggest several ways to provide quantitative validation of your claim.
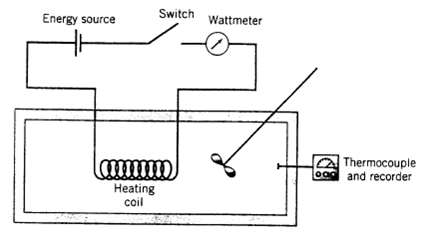
Energy source Switch (I!!!!!!! Heating coil Wattmeter Thermocouple og and recorder
Step by Step Solution
3.31 Rating (166 Votes )
There are 3 Steps involved in it
a The gas temperature remains constant while the circuit is open If heat losses c... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
13-E-C-E-C-P (370).docx
120 KBs Word File


