Question: How many types of nonequivalent protons are present in each of the followingmolecules? (c) (b) CH3CH2CH20CH3 (a) H3C CH3 Naphthalene (e) (d) C=CH2 CO2CH2CH3 Ethyl
How many types of nonequivalent protons are present in each of the followingmolecules?
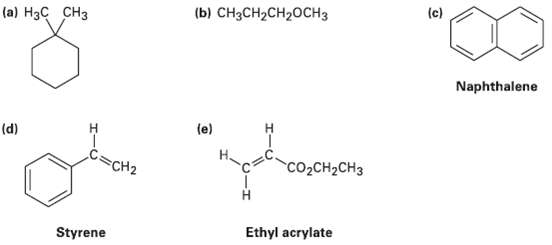
(c) (b) CH3CH2CH20CH3 (a) H3C CH3 Naphthalene (e) (d) C=CH2 CO2CH2CH3 Ethyl acrylate Styrene
Step by Step Solution
3.36 Rating (174 Votes )
There are 3 Steps involved in it
a 1 c Compound e 1 H3C CH3 2 3 2 pa 2 ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
22-C-O-S (210).docx
120 KBs Word File


