Question: How strong a base would you expect a Grignard reagent to be? Look at Table 8.1, and then predict whether the following reactions will occur
How strong a base would you expect a Grignard reagent to be? Look at Table 8.1, and then predict whether the following reactions will occur as written.?(The pKa of NH3 is 35.)
(a) CH3MgBr + H ? C ? C ? H ? CH4 + H ? C ? C ? MgBr
(b) CH3MgBr + NH3 ? CH4 + H2N ? MgBr
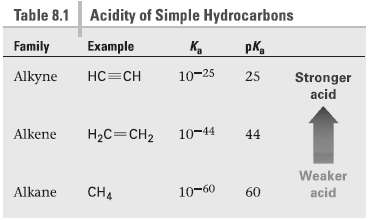
Acidity of Simple Hydrocarbons Table 8.1 Family Example K, pK, 10-25 Alkyne 25 Stronger acid 10-44 Alkene H2C=CH2 44 Weaker 10-60 CH4 Alkane 60 acid
Step by Step Solution
3.45 Rating (155 Votes )
There are 3 Steps involved in it
Table 81 shows that the pK of CH3H is 60 Since CH4 is a ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
22-C-O-O-H (9).docx
120 KBs Word File


