Question: An immersed electrical heater is used to raise the temperature of a liquid from 20?C to 60?C in 20.0 mm. The combined mass of the
An immersed electrical heater is used to raise the temperature of a liquid from 20?C to 60?C in 20.0 mm. The combined mass of the liquid and the container is 250 kg, and the mean heat capacity of the system is 4.00 kJ/ (kg??C). The liquid decomposes explosively at 85?C. At 10.00 a.m. a batch of liquid is poured into the vessel and the operator turns on the heater and leaves to make a phone call. Ten minutes later, his supervisor walks by and looks at the strip chart record of the power input. This what she sees, the supervisor immediately shuts off the heater and charges off to pass on to the operator several brief observations that come to her mind.
(a) Calculate the required constant power input Q (kW), neglecting energy losses from the container.
(b) Write and integrate using Simpson?s rule (Appendix A.3) an energy balance on the system to estimate the system temperature at the moment the heater is shut off. Use the following data from the recorder chart:
(c) Suppose that if the heat had not been shut off, Q would have continued to increase linearly at a rate of 10kW/min. At what time would ever one in the plant realize that something was wrong?
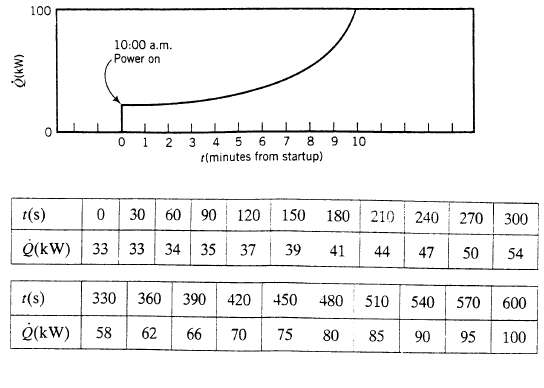
(kW) 100 10:00 a.m. Power on 1 1 1 1 0 1 2 3 4 5 6 7 8 9 10 r(minutes from startup) 0 30 60 90 120 150 39 t(s) Q(kW) 33 33 34 35 37 t(s) Q(kW) 58 62 80 | 210 41 44 47 330 360 390 420 450 480 510 266 70 75 80 85 240 270 300 50 54 540 570 600 90 95 100
Step by Step Solution
3.57 Rating (175 Votes )
There are 3 Steps involved in it
a Integral energy balance 10 to 1 20 min QAU MC AT 250 kg 400 ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
13-E-C-E-C-P (611).docx
120 KBs Word File


