Question: Two posssible Lewis structures for the molecule CH,S are given. Determine the formal charge on each atom in both structures. Answer Bank S: +2
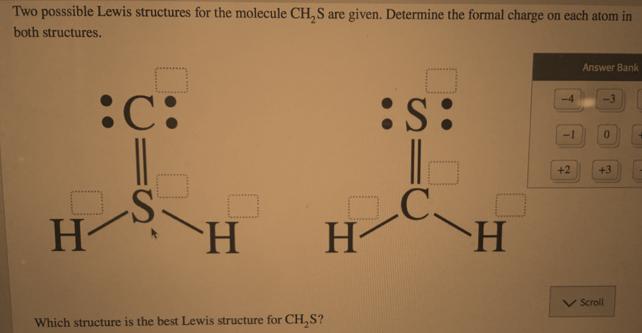
Two posssible Lewis structures for the molecule CH,S are given. Determine the formal charge on each atom in both structures. Answer Bank S: +2 +3 H-C V Scroll Which structure is the best Lewis structure for CH,S?
Step by Step Solution
3.35 Rating (161 Votes )
There are 3 Steps involved in it
To determine the formal charges in the provided Lewis structures for CHS we need to use the formula ... View full answer

Get step-by-step solutions from verified subject matter experts


