Question: In Rutherford??s famous scattering experiments that led to the planetary model of the atom, alpha particles (having charges of +2e and masses of 6.64 x
In Rutherford??s famous scattering experiments that led to the planetary model of the atom, alpha particles (having charges of +2e and masses of 6.64 x 10??27 kg) were fired toward a gold nucleus with charge +79e. An alpha particle, initially very far from the gold nucleus, is fired at 2.00 x 107 m/s directly toward the nucleus, as in figure. How close does the alpha particle get to the gold nucleus before turning around? Assume the gold nucleus remainsstationary.
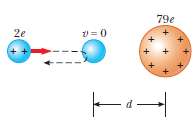
79e 2e 2 = 0 -d-
Step by Step Solution
3.25 Rating (163 Votes )
There are 3 Steps involved in it
From conservation of energy KE PE ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
63-P-E-C-D (465).docx
120 KBs Word File


