Question: Irreversible second-order reaction in an agitated tank consider a system similar to that discussed in Problem 23B.4, except that the solute disappears according to a
Irreversible second-order reaction in an agitated tank consider a system similar to that discussed in Problem 23B.4, except that the solute disappears according to a second-order reaction; that is, RA,tot = – k'''2 Vc2A. Develop an expression for c A as a function of time by the following method:
(a) Use a macroscopic mass balance for the tank to obtain a differential equation describing the evolution of CA with time.
(b) Rewrite the differential equation and the accompanying initial condition in terms of the variable the nonlinear differential equation obtained in this way is a Bernoulli differential equation.
(c) Now put v = 1/u and perform the integration. Then rewrite the result in terms of the original variable CA.
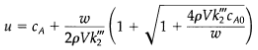
4pVk"c Ao | 2pVk"
Step by Step Solution
3.36 Rating (168 Votes )
There are 3 Steps involved in it
Irreversible secondorder reaction in an agitated tank a The unsteady state mass balance on substance ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
6-E-C-E-T-P (342).docx
120 KBs Word File


