Question: It has been proposed that oxygen be separated from nitrogen by absorbing and desorbing air in water. Pressures from 101.3 to 10,130 kPa and temperatures
It has been proposed that oxygen be separated from nitrogen by absorbing and desorbing air in water. Pressures from 101.3 to 10,130 kPa and temperatures between 0 and 100°C are to be used.
(a) Devise a workable scheme for doing the separation assuming the air is 79 mol% N2 and 21 mol% O2.
(b) Henry's law constants for O2 and N2 are given in Figure. How many batch absorption steps would be necessary to make 90 mol% pure oxygen? What yield of oxygen (based on total amount of oxygen feed) would beobtained?
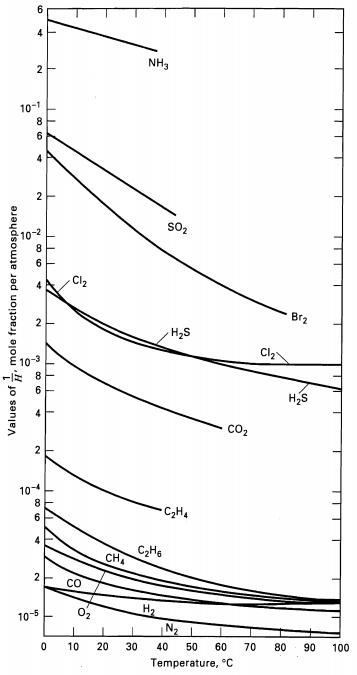
NH3 2 10-1 sO2 Cl2 Br2 H2S Cl2 10-3 H2s CO2 104 8. C,H4 6. CH6 CH CO H2 N2 10-5 10 20 30 40 50 60 70 80 90 100 Temperature, C 2. 4, 2. 4, 2. Values of , mole fraction per atmosphere
Step by Step Solution
3.59 Rating (167 Votes )
There are 3 Steps involved in it
Henrys law constants are given in Fig as 1H as function of temperature where from Eq 432 x i 1H i y i P with pressure in atm Absorption is most efficient at high pressures while desorption is best at ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
37-E-C-E-S-P (152).docx
120 KBs Word File


