Question: It is proposed that oxygen be separated from nitrogen by absorbing and desorbing air in water. Pressures from 101.3 to 10,130 kPa and temperatures between
It is proposed that oxygen be separated from nitrogen by absorbing and desorbing air in water. Pressures from 101.3 to 10,130 kPa and temperatures between 0 and 100οC are to be used.
(a) Devise a scheme for the separation if the air is 79 mol% N2 and 21 mol% O2.
(b) Henry’s law constants for O2 and N2 are given in Figure 4.26. How many batch absorption steps would be necessary to make 90 mol% oxygen? What yield of oxygen (based on the oxygen feed) would be obtained?
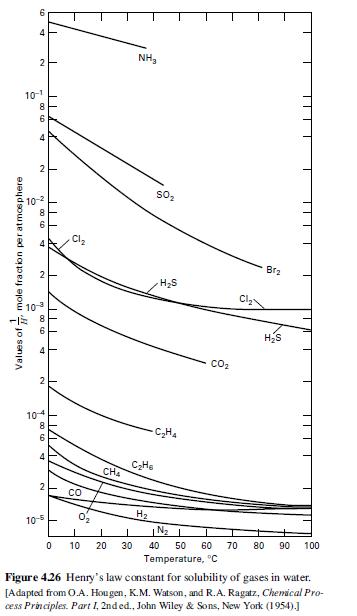
4 Values of mole fraction per atmosphere 2 10-1 -00 to 8 6 4 2 y 00 co -10-2 st N m00 CD 10 -18 6 st 2 104 8 6 4 IL 10-5 I 2 CO Cl 0 CHA NH CH 0, I I 10 20 30 H 50 HS CH CO Brz HS IN 1 40 50 60 70 80 Temperature, C 11 L 90 100 Figure 4.26 Henry's law constant for solubility of gases in water. [Adapted from O.A. Hougen, K.M. Watson, and R.A. Ragatz, Chemical Pro- cess Principles. Part I, 2nd ed., John Wiley & Sons, New York (1954).]
Step by Step Solution
3.44 Rating (170 Votes )
There are 3 Steps involved in it
Answer a A scheme for the separation is as follows 1 Charge the column with air at 10... View full answer

Get step-by-step solutions from verified subject matter experts


