Question: A laboratory apparatus to measure the diffusion coefficient of vapor-gas mixtures consists of a vertical, small-diameter column containing the liquid phase that evaporates into the
A laboratory apparatus to measure the diffusion coefficient of vapor-gas mixtures consists of a vertical, small-diameter column containing the liquid phase that evaporates into the gas flowing over the mouth of the column. The gas flow rate is sufficient to maintain a negligible vapor concentration at the exit plane. The column is 150 mm high, and the pressure and temperature in the chamber are maintained at 0.25 atm and 320 K, respectively. For calibration purposes, you've been asked to calculate the expected evaporation rate (kg/h ? m2) for a test with water and air under the foregoing conditions, using the known value of DAB for the vapor-air mixture.
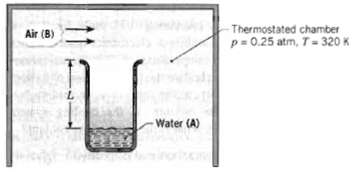
-Thermostated chamber p= 0.25 atm, T-320 K !! Air (B) Water (A)
Step by Step Solution
3.51 Rating (174 Votes )
There are 3 Steps involved in it
KNOWN Column containing liquid phase of water A evaporates into the air B flowing over the mouth of ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
8-E-M-E-H-M-T (1364).docx
120 KBs Word File


