Question: Nitric acid is produced commercially by the Ostwald process. In the first step, ammonia is oxidized to nitric oxide: 4NH 3 (g) + 5O 2
Nitric acid is produced commercially by the Ostwald process. In the first step, ammonia is oxidized to nitric oxide:
4NH3(g) + 5O2(g) → 4NO(g) + 6H2O(g)
Assume this reaction is carried out in the apparatus diagramed below.
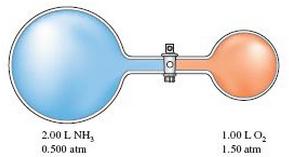
The stopcock between the two reaction containers is opened, and the reaction proceeds using proper catalysts. Calculate the partial pressure of NO after the reaction is complete. Assume 100% yield for the reaction, assume the final container volume is 3.00 L, and assume the temperature is constant.
2.00 L NH3 0.500 atm 1.00 LO2 1.50 atm
Step by Step Solution
3.43 Rating (156 Votes )
There are 3 Steps involved in it
The actual ratio is larger than the required ratio so NH 3 in the denominator is limiting Because ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
442-C-P-c-G (48).docx
120 KBs Word File


