Question: Nitrite (NO 2 - ) can be determined by oxidation with excess Ce 4+ , followed by back titration of the unreacted Ce 4+ .
Nitrite (NO2-) can be determined by oxidation with excess Ce4+, followed by back titration of the unreacted Ce4+. A 4.030-g sample of solid containing only NaNO2 (FM 68.995) and NaNO3 was dissolved in 500.0 mL. A 25.00-mL sample of this solution was treated with 50.00 mL of 0.118 6 M Ce4+ in strong acid for 5 min, and the excess Ce4+ was back-titrated with 31.13 mL of 0.042 89 M ferrous ammonium sulfate.
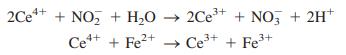
Calculate the weight percent of NaNO2 in the solid.
2Ce** + NO, + H,0 2Ce* + NO, + 2H* Ce*+ + Fe2+ - Ce* + Fe+
Step by Step Solution
3.41 Rating (179 Votes )
There are 3 Steps involved in it
5000 mL of 0118 6 M Ce 5930 mmol Ce4 3113 mL of 0042 89 MFe... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
878-E-C-E-E-C (2088).docx
120 KBs Word File


