Question: One frequently used method for preparing methyl esters is by reaction of carboxylic acids with diazomethane, CH2N2. The reaction occurs in two steps: (1) Protonation
One frequently used method for preparing methyl esters is by reaction of carboxylic acids with diazomethane, CH2N2. The reaction occurs in two steps: (1) Protonation of diazomethane by the carboxylic acid to yield methyldiazonium ion, CH3N2+, plus a carboxylate ion; and (2) reaction of the carboxylate ion with CH3N2+.
(a) Draw two resonance structures of diazomethane, and account for step 1.
(b) What kind of reaction occurs in step2?
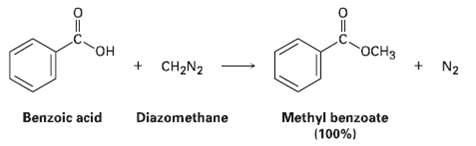
OCH3 + N2 + CH2N2 Methyl benzoate (100%) Benzoic acid Diazomethane
Step by Step Solution
3.41 Rating (170 Votes )
There are 3 Steps involved in it
a HCNEN HCNN Resonance forms show that the carbon of diazo... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
22-C-O-CA (155).docx
120 KBs Word File


