Phenol, C6H5OH, is a stronger acid then methanol, CH3OH, even though both contains an O ? H
Question:
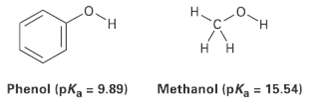
Phenol, C6H5OH, is a stronger acid then methanol, CH3OH, even though both contains an O ? H bond. Draw the structures of the anions resulting from loss of H+ from phenol and methanol, and use resonance structures to explain the difference in acidity.
Transcribed Image Text:
H. H. Methanol (pKa = 15.54) = 9.89) Phenol (pK
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 76% (13 reviews)
H 66666 HH When p...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Related Video
In this video, A mixture of methanol and air in a large polycarbonate bottle is ignited. The resulting rapid combustion reaction, often accompanied by a dramatic ‘whoosh’ sound and flames, demonstrates the large amount of chemical energy released in the combustion of alcohol
Students also viewed these Organic Chemistry questions
-
Explain the following observations: (a) HNO3 is a stronger acid than HNO2; (b) H2S is a stronger acid than H2O; (c) H2SO4 is a stronger acid than HSO4-; (d) H2SO4 is a stronger acid than H2SeO4; (e)...
-
Explain the following observations: (a) HCl is a stronger acid than H2S; (b) H3PO4 is a stronger acid than H3AsO4; (c) HBrO3 is a stronger acid than HBrO2; (d) H2C2O4 is a stronger acid than HC2O4-;...
-
Use resonance formulas to explain why polyacetylene has delocalized molecular orbitals extending over the length of the molecule, whereas the following molecule does not. HHHHH
-
Why should one-time write-offs of fixed capital be used in absorption cost systems?
-
Samuel and Annamaria are married, file a joint return, and have three qualifying children. In 2016, they earn wages of $34,000 and no other income. Determine the amount of their earned income credit...
-
State with reason whether the following statements are true, false, or uncertain. Be precise. a. The t test of significance discussed in this chapter requires that the sampling distributions of...
-
For each of the following situations, calculate the population standard error of the mean \(\sigma \mathrm{X}^{-}\). a. \(\sigma=8 ; N=16\) b. \(\sigma=12 ; N=64\) c. \(\sigma=2 ; N=25\) d....
-
In this problem, you will see why the Equity Premium Puzzle described in Application 4.5 really is a puzzle. Suppose that a person with $100,000 to invest believes that stocks will have a real return...
-
Braxton Corporation has the following data as of December 31, 2024: Total Current Liabilities Total Current Assets Long-term Liabilities $ 61,420 Total Stockholders' Equity 43,600 Other Assets...
-
Ceramic Structures has experienced rapid growth over the past several years. Sales are expected to grow at 15% per year for the next three years. Sales growth has been fueled by aggressive pricing as...
-
We?ll see that organic molecules can be classified according to the functional groups they contain, where a functional group is a collection of atoms with a characteristics chemical reactivity. Use...
-
Monobromination of toluene gives a mixture of three bromotoluene products. Draw and name them.
-
On January 1, 2014, Woodrow Company purchased 30 percent of the outstanding common shares of Trevor Corporation at a total cost of $ 560,000. Management intends to hold the stock for the long term....
-
Within each of the six model nations, what particular historical developments have had a major effect on their formation of criminal law and criminal justice administration?
-
What are the types of accounting fraud? Discuss the accounting fraud of Countrywide.
-
What is the difference between Criminal and Civil law? Provide a real-world example of a dispute that would be classified as civil law and one that could be classified as criminal law. Please list...
-
Always Stayed Focus The new Audi A3. Discuss the following question? How does the advertisement attempt to get your attention? What is it an advertisement for? Who is the target audience for this...
-
Describe critical theories related to cultural competency and diversity in organizations. Express personal critical approaches to cultural competency and diversity. Explain.
-
Do you think Congress can devise a law that will pass constitutional muster to protect children from Internet pornography? What suggestions do you have in this area?
-
Write a paper by answer the following question: Should Recycling Be Mandatory?
-
Using the Redlich-Kwong equation of state, compute the following quantities for nitrogen at 298.15 K. a. The difference C P C V as a function of pressure from low pressures to very high pressures b....
-
Arrange these compounds in order of increasing SN2 reaction rate: CI Br CI Br
-
Arrange these compounds in order of increasing SN1 reaction rate: Ph-Br Br + Br CI
-
Show the products of these reactions and explain whether each would follow an SN1 or an SN2 mechanism: a) C) B CI + OH Br + SH DMF CHOH HO Br + HO CHOH HO b) d) f) CI + HO CH,OH OTS + CH0 Br + CHCO...
-
You are a supervisor at your company. You've overheard Employee A making offensive comments to other employees, and Employee B has complained about these comments. You've given Employee A a verbal...
-
What is the probability of developing and dying from cancer today?
-
On January 1, 2024, Majestic Mantles leased a lathe from Equipment Leasing under a finance lease. Lease payments are made annually. Title does not transfer to the lessee and there is no purchase...

Study smarter with the SolutionInn App


