Reaction of the following S tosylate with cyanide ion yields a nitrile product that also has S
Question:
Reaction of the following S tosylate with cyanide ion yields a nitrile product that also has S stereochemistry.Explain.
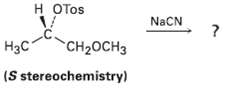
Transcribed Image Text:
н ОTOS NaCN |Нас" CH2OCH3 (S stereochemistry)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 45% (11 reviews)
According to CahnIngoldPrelog rules Section 65 the nucleophile ...View the full answer

Answered By

Branice Buyengo Ajevi
I have been teaching for the last 5 years which has strengthened my interaction with students of different level.
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Show the product of the Diels?Alder reaction of the following diene with 3-buten-2-one, H 2 C = CHCOCH 3 . Make sure you show the full stereochemistry of the reaction product.
-
Show the major product(s) from reaction of the following substances with (i) CH3CH2Cl, AlCl3 and (ii) HNO3,H2SO4 (b) (a)
-
Determine the tax year(s) each of the following S corporations must use. Explain. a. Will, Dan, and Tom are equal owners of Rheen Corporation, and each has a different fiscal year. Will has a fiscal...
-
The income statement for Performance Limited is given below along with some supplementary information. Performance Limited Income Statement for the year ended December 30, 2020 (in $000s) Division A...
-
How has the Container Store's hiring practices helped to create their success?
-
(a) Arrange the following compounds in order of decreasing acidity and explain your answer: CH3CH2NH2, CH3CH2OH, and CH3CH2CH3. (b) Arrange the conjugate bases of the acids given in part (a) in order...
-
Proper recording within general ledger control accounts does not necessarily mean that transactions are posted accurately to individual vendor accounts. What controls can management institute to...
-
Willis Music Co. advertised a television set at $22.50 in the Sunday newspaper. Ehrlich ordered a set, but the company refused to deliver it on the grounds that the price in the newspaper ad was a...
-
On January 1, 2020, Stream Company acquired 20 percent of the outstanding voting shares of Q-Video, Inc., for $702,000. Q-Video manufactures specialty cables for computer monitors. On that date,...
-
The Architect total contract fee for the project is: $235,000. The Basic Services will be performed in four phases as described below. The services shall be rendered in the following phases:...
-
(R)-2-Bromooctanc undergoes racemization to give () -2-bromooctane when treated with NaBr in dimethyl sulfoxide. Explain.
-
Ethers can often be prepared by S N 2 reaction of alkoxide ions, RO ? , with alkyl halides. Suppose you wanted to prepare cyclohexyl methyl ether. Which of the two possible routes shown below would...
-
Find the function and graph it for a function of the form y = 3 sin(x + c) that passes through (0.25, 0) and for which c has the smallest possible positive value.
-
A scale reads 264 N when a piece of iron is hanging from it. What does it read (in N) when it is lowered so that the iron is submerged in water? Please give your answer in N 2. A boat (with a flat...
-
1. A modern-day zeppelin holds 9,840 m 3 of helium. Compute its maximum payload at sea level. (Assume the helium and air to be at 0C and 1 atm.) Please give your answer in N 2. An empty storage tank...
-
A 0.640-kg ball is dropped from rest at a point 2.70 m above the floor. The ball rebounds straight upward to a height of 1.79 m. Taking the negative direction to be downward, what is the impulse of...
-
A coin of mass 10g placed 24cm from the centre of a rotating, horizontal turntable slips when its speed is 80 cm/s. What is the coefficient of static friction between coin and turntable? Provide your...
-
The temperature coefficient of resistivity of a material is 0.0040 (C o ) -1 . By how much T does the temperature of a wire made from this material have to change, in order that the resistance of the...
-
At what speed relative to a laboratory does a clock tick at half the rate of an identical clock at rest in the laboratory? Give your answer as a fraction of \(c\).
-
Selected condensed data taken from a recent statement of financial position of Morino Ltd. are as follows. MORINO LTD. Statement of Financial Position (partial) Other current assets...
-
Consider the reaction: In a reaction mixture at equilibrium, the partial pressure of NO is 108 torr and that of Br 2 is 126 torr. What is the partial pressure of NOBr in this mixture? 2 NO(g) + Br(g)...
-
Grignard reagents add to carbonate esters as they add to other esters. (a) Predict the major product of the following reaction. (b) Show how you would synthesize 3-ethylpentan-3-ol using diethyl...
-
One mole of acetyl chloride is added to a liter of triethylamine, resulting in a vigorous exothermic reaction. Once the reaction mixture has cooled, 1 mole of ethanol is added. Another vigorous...
-
Show how you would accomplish the following multistep syntheses, using the indicated starting material and any necessary reagents. (a) hept-6-en-1-ol e-caprolactone (b) methoxybenzene...
-
Give a summary of concepts and the applications for the following quistion: When a 200.0-g mass is attached to a spring, it stretches the spring by 7.50 cm. With that mass-spring system in...
-
A potato is launched out of a building (building A) from the 10th floor which is 15 m high, with a velocity of 55 m/s at an angle of 35.0 above the horizontal. There is a building (building 2) 130 m...
-
An eagle is flying horizontally at a speed of 2.9 m/s when the fish in her talons wiggles loose and falls into the lake 4.4 m below. Calculate the magnitude of the velocity of the fish relative to...

Study smarter with the SolutionInn App


