Question: A sample consisting of 1 mol of perfect gas atoms (for which CVm = 3/2 R) is taken through the cycle shown in Fig. 2.34.
A sample consisting of 1 mol of perfect gas atoms (for which CV•m = 3/2 R) is taken through the cycle shown in Fig. 2.34.
(a) Determine the temperature at the points 1, 2, and 3.
(b) Calculate q, w, ∆U, and ∆H for each step and for the overall cycle. If a numerical answer cannot be obtained from the information given, then write in +, -, 0, or? As appropriate
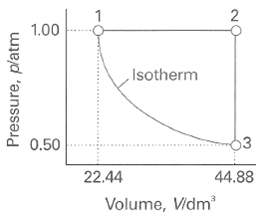
1.00 ,Isotherm 13 0.50 22.44 44.88 Volume, Vidm' Pressure, platm
Step by Step Solution
3.22 Rating (160 Votes )
There are 3 Steps involved in it
The temperatures are readily obtained from the perfect gas equation T PV nR 100 atm x 224 dm 100 mol ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
57-C-PC-E (38).docx
120 KBs Word File


