Question: Show for a photon gas that: (a) (b) d j /dV = j /3V (c) p = U/3V Thus the radiation pressure is equal
Show for a photon gas that:
(a)
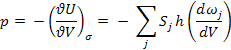
(b) dωj/dV = –ωj/3V
(c) p = U/3V
Thus the radiation pressure is equal to 1/3 × (energy density).
(d) Compare the pressure of thermal radiation with the kinetic pressure of a gas of H atoms at a concentration of 1 mole cm-3 characteristic of the Sun. at what temperature (roughly) are the two pressures equal? The average temperature of the sun is believed to be near 100 mole cm-3at the center, where the kinetic pressure is considerably higher than the radiation pressure.
dw; - - ( dv lov.
Step by Step Solution
3.55 Rating (165 Votes )
There are 3 Steps involved in it
a b We assume an isotropic volume change of a cubeshape cavity From 15 c Insert 51 into 50 d Ins... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
42-P-S-S-T-T (23).docx
120 KBs Word File


