Question: Solids soaked with liquid hexane are dried by being contacted with nitrogen at an elevated temperature. The gas stream leaving the dryer is at 80?C,
Solids soaked with liquid hexane are dried by being contacted with nitrogen at an elevated temperature. The gas stream leaving the dryer is at 80?C, 1 atm absolute and 50% relative saturation.
(a) One of several possibilities for recovering the hexane from the gas is to send the stream to a cooling condenser. The gas stream leaving the condenser would contain 5.00mole% hexane, and hexane condensate would be recovered at a rate of 1.50k mol/min. The condenser would be operated at a pressure of 1 atm absolute. Calculate the temperature to which the gas must be cooled and the required flow rate of fresh nitrogen to the dryer in standard cubic meters per minute (SCMM).
(b) In an alternative arrangement, the gas leaving the dryer would be compressed to 10.0 atm and the temperature would simultaneously be increased so that the relative saturation remains at 50%. The gas then would be cooled at constant pressure to produce a stream containing 5.00mole% hexane. Calculate the final gas temperature and the ratio of volumetric flow rates of the gas streams leaving and entering the condenser. State any assumptions you make.
(c) What would you need to know to determine which of processes (a) and (b) is more cost- effective?
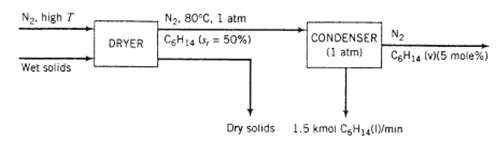
N. high T Wet solids N, 80C, 1 atm DRYER CH14 (s, = 50%) Dry solids CONDENSER (1 atm) N C6H14 (V)(5 mole %) 1.5 kmol CH14(l/min
Step by Step Solution
3.59 Rating (167 Votes )
There are 3 Steps involved in it
Let Hnhexane a no kmolmin yo kmol Hvkmol 1yo kmol Nkmol 80C 1 atm 50 rel sat n Condenser kmolmin 005 ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (2 attachments)
13-E-C-E-C-P (275).pdf
180 KBs PDF File
13-E-C-E-C-P (275).docx
120 KBs Word File


