In Example 22.9, the secondary combustion reaction of oxylene was ignored. If this reaction can be approximated
Question:
In Example 22.9, the secondary combustion reaction of oxylene was ignored. If this reaction can be approximated by the following reaction rate, rework the example for inlet partial pressures of o-xylene (Component A) of 0.015, 0.016, 0.0170, and 0.0175 bar.![C8 H10+2O8CO2 +5HO o-xylene -r [mol/h/mcat] -" A = 2 102 e 25,000/T[K]p [bar] AH comb = 4375 kJ/mol](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1699/4/4/1/667654b6c03a33891699441665667.jpg)
Do the results change significantly from those given in Example 22.9? Are your conclusions surprising, and could you have determined these results by simply comparing the basic kinetic equations?
Problem 22.9
Consider the production of cumene (c) from the catalytic alkylation reaction of benzene (b) using propylene (p)— Reaction 1. A second undesirable reaction (Reaction 2) between propylene and cumene occurs to produce pdiisopropyl benzene (DIPB):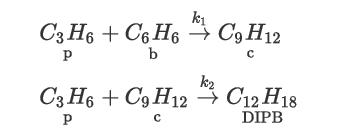
where r1 = k1 cp cb and r2 = k2 cp cb and the rates of reaction are given in mol/L/s and the rate constants are![k = 2.8 x 107 exp = 2.32 x 10 exp k2= 12,530 T[K] and 17,650 170). These reactions are quite](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1699/4/4/1/388654b6aecae9931699441387138.jpg)
exothermic, and typical reactor temperatures are in the range 350°C to 410°C. The heats of reaction per mol of product in this temperature range are −100.6 kJ/mol and 121.3 kJ/mol for Reactions 1 and 2, respectively. The heat capacities for the reactant and product streams may be taken to be the same and equal to 2.44 kJ/kg/K in this range. For this system, do the following:
1. Determine the conversion of an equimolar feed of propylene and benzene (feed conditions are 350°C, 3 MPa, and 100 mol/s) if the reactor is run as an adiabatic packed bed with a maximum outlet temperature of 425°C.
2. Based on the results from Part (a), is it feasible to obtain an overall conversion of 80% of the benzene using a series of staged packed beds with cooling between stages to bring the temperature back to 350°C at the inlet of the next reactor stage? In practice, no more than four stages would be used.
Step by Step Answer:

Analysis Synthesis And Design Of Chemical Processes
ISBN: 9780134177403
5th Edition
Authors: Richard Turton, Joseph Shaeiwitz, Debangsu Bhattacharyya, Wallace Whiting





