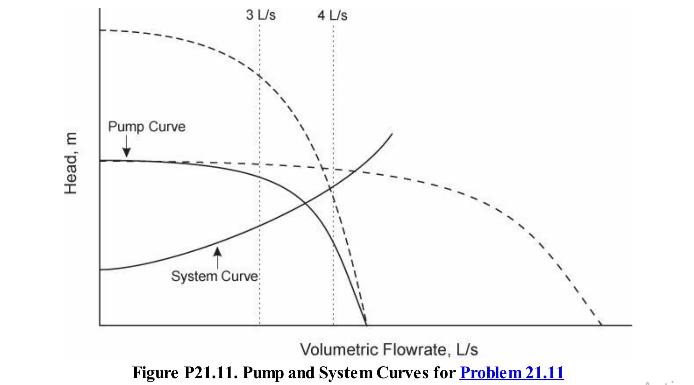
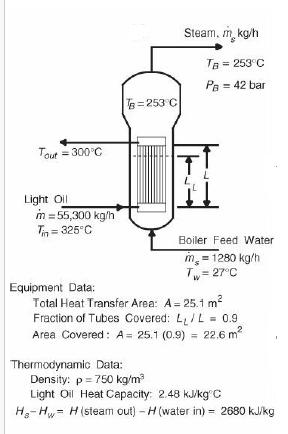
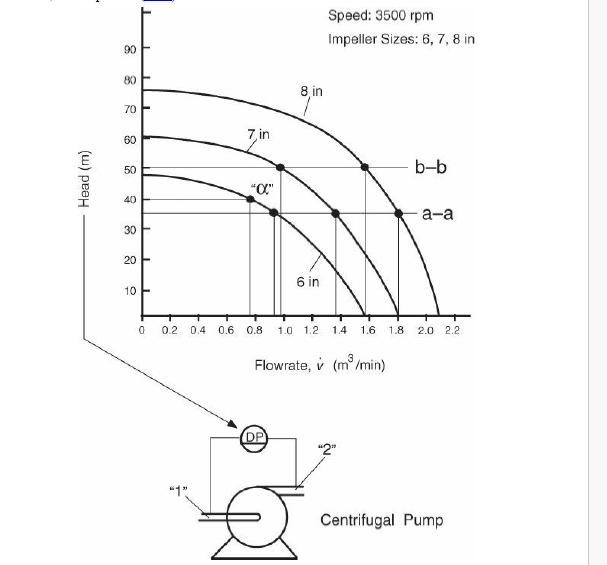
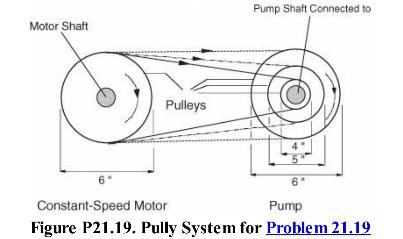
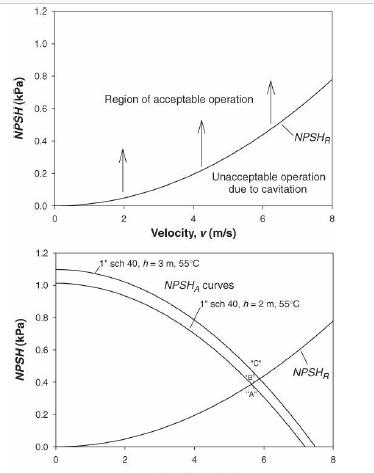
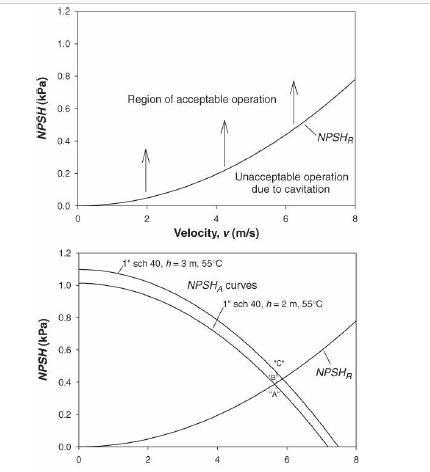
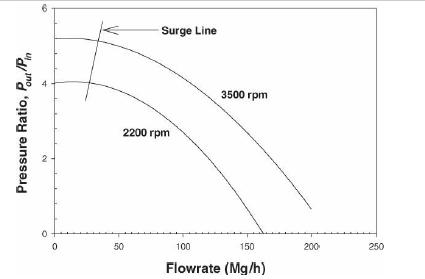
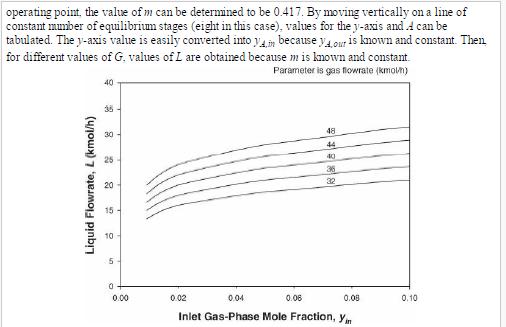
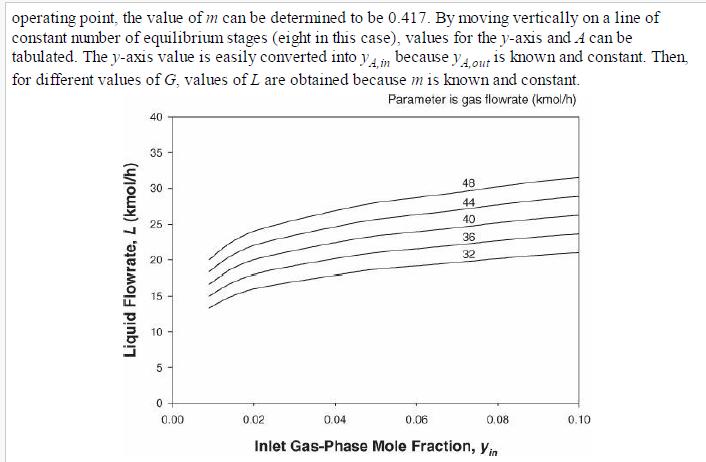
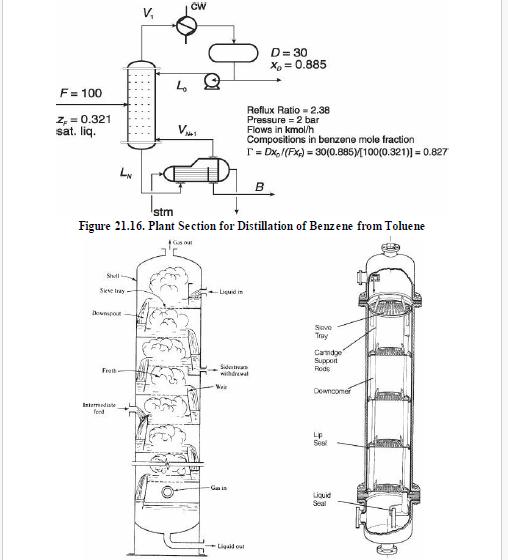
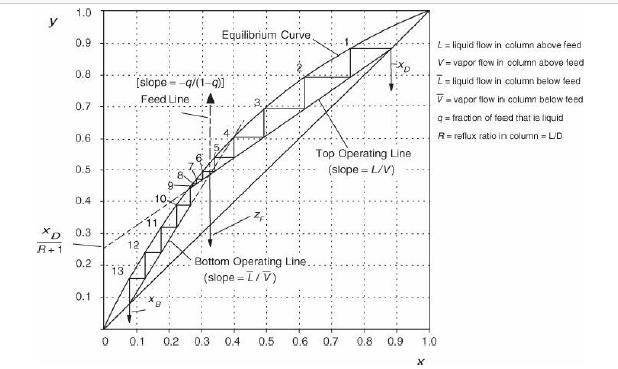
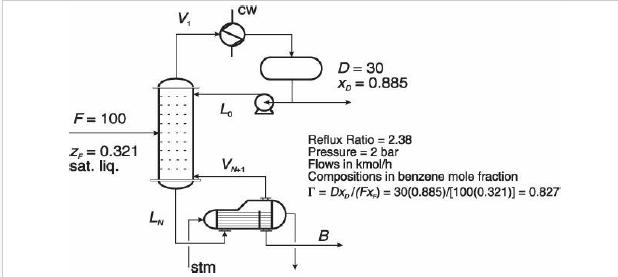
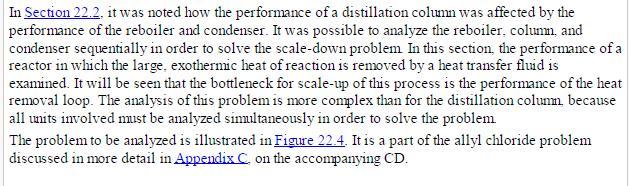
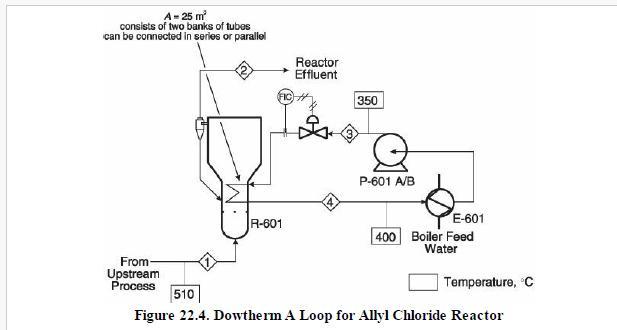
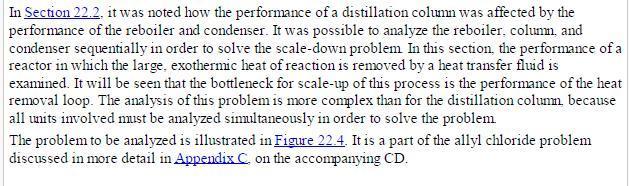
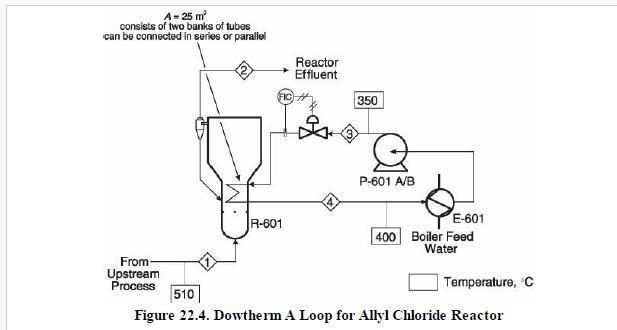
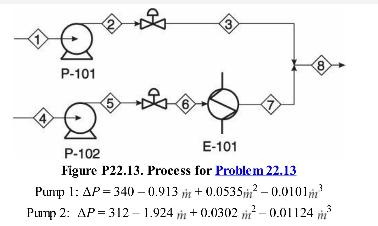
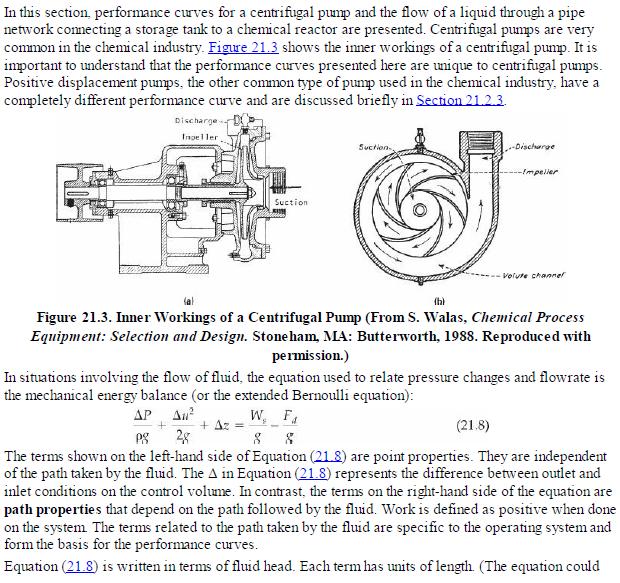
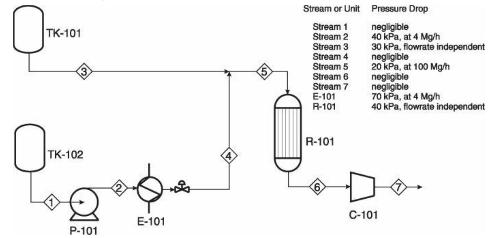
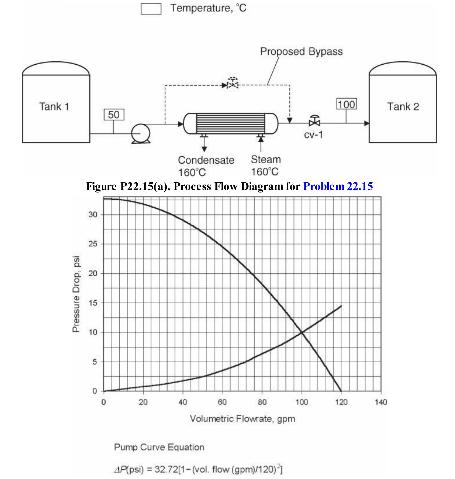
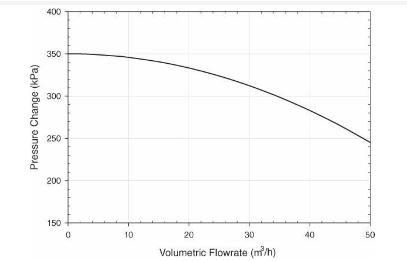
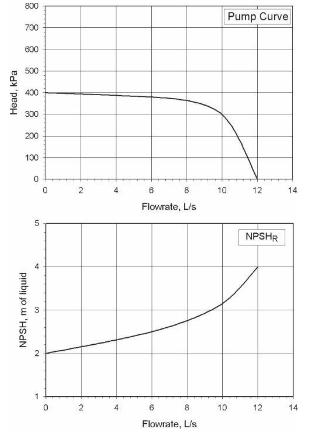
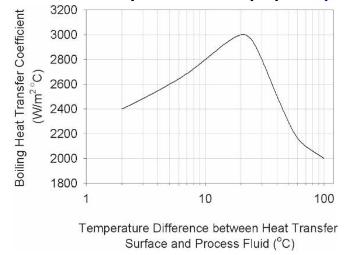
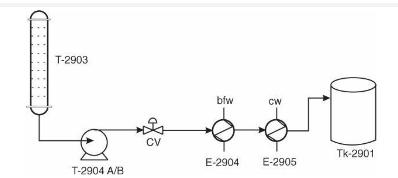
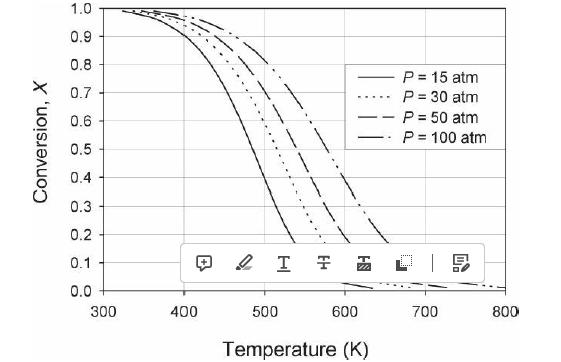
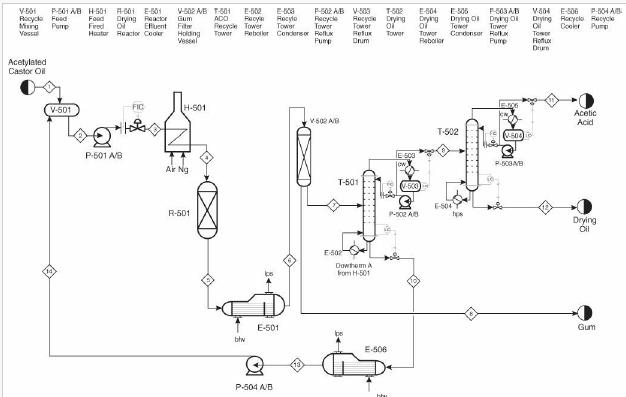
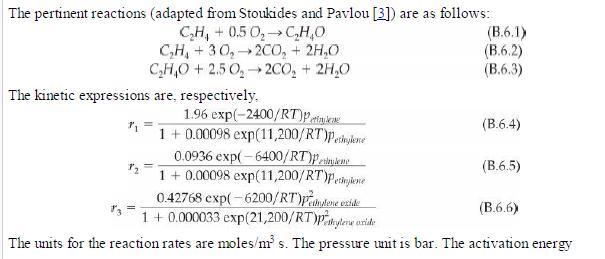
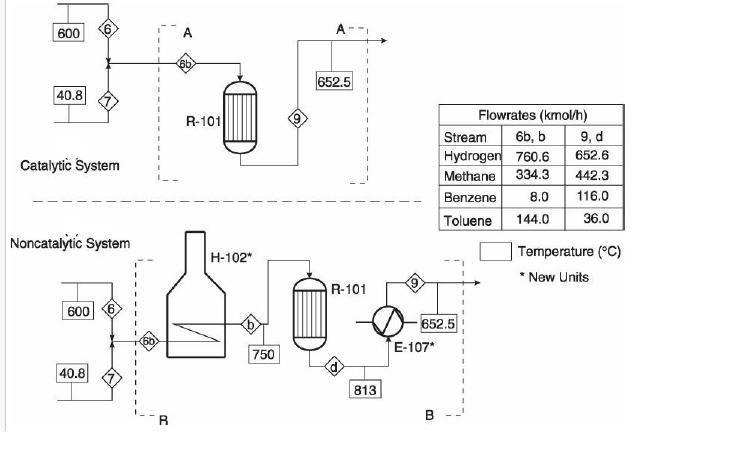
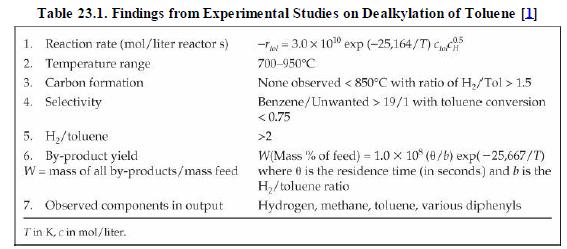
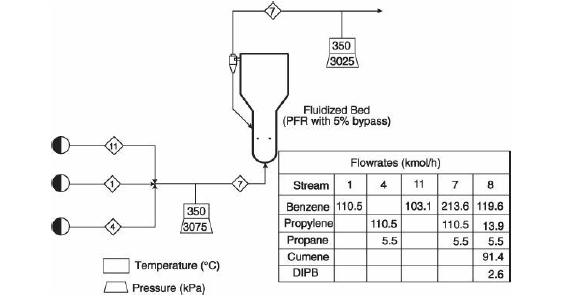
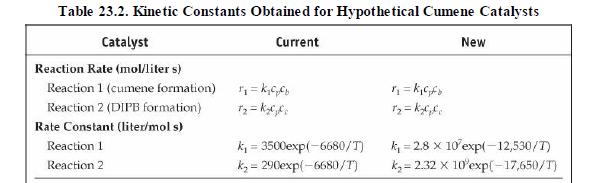
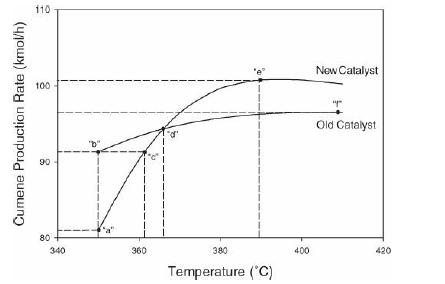
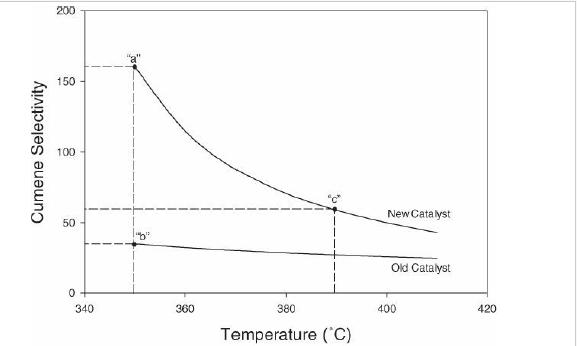
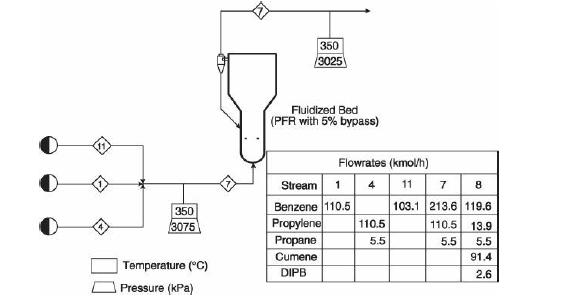
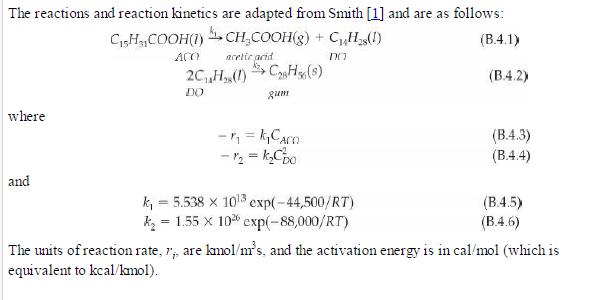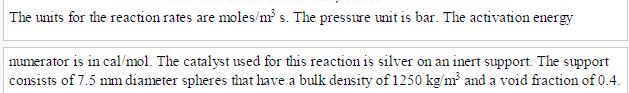Analysis Synthesis And Design Of Chemical Processes 4th Edition Richard Turton, Richard C. Bailie, Wallace B. Whiting, Joseph A. Shaeiwitz, Debangsu Bhattacharyya - Solutions
Discover comprehensive insights and solutions with the "Analysis, Synthesis, and Design of Chemical Processes, 4th Edition" by Richard Turton and colleagues. Access an extensive collection of solved problems and step-by-step answers with our online solution manual. Enhance your understanding with chapter solutions and instructor manuals, designed for effective learning and teaching. Our answers key provides in-depth explanations, making it a valuable resource for both students and instructors. Explore the test bank and questions and answers section for thorough preparation. Download solutions in PDF format for free and elevate your study experience with this essential textbook.
![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()