Reduce the amount of hydrogen in the feed to the reactor to the stoichiometric amountthat is, 144
Question:
Reduce the amount of hydrogen in the feed to the reactor to the stoichiometric amount—that is, 144 kmol/h—and determine the effect on the equilibrium conversion at 600°C.
Example 6.3
For the PFD presented in Figure 1.5,
1. Calculate the actual conversion.
2. Evaluate the equilibrium conversion at 600°C.
Assuming ideal gas behavior: K = (Nbenzene Nmethane)/(NtolueneNhydrogen) where N represents the Information on the feed stream to the reactor from Table 1.5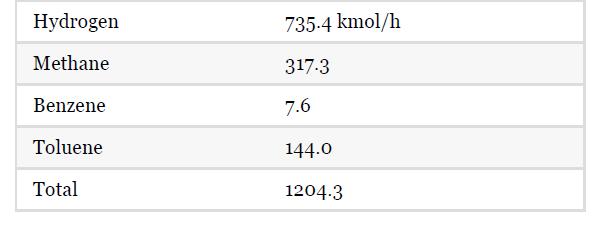
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Analysis Synthesis And Design Of Chemical Processes
ISBN: 9780134177403
5th Edition
Authors: Richard Turton, Joseph Shaeiwitz, Debangsu Bhattacharyya, Wallace Whiting
Question Posted:





