When coal is burned to form synthesis gas (syngas), which contains mostly CO, H 2 , H
Question:
When coal is burned to form synthesis gas (syngas), which contains mostly CO, H2, H2S, and CO2, the H2S must be removed. The gas is called syngas because H2 and CO are the building blocks for synthesis of hydrocarbons. One method for removal of H2 S is the Selexol™ solvent, in which the H2S is highly soluble. The Selexol solvent is the dimethyl ether of polyethylene glycol and may be assumed to have a molecular weight of 300 kg/kmol, μ = 2.5 × 10−3 kg/m s, and ρ = 900 kg/m3.
Consider the removal of H2S only from an otherwise inert mixture of gases; that is, none of the other gases are soluble in the Selexol solvent. The partition coefficient for H2S between gas and solvent is given by the relationship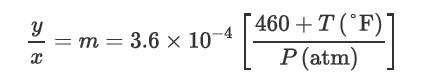
The problem at hand is the removal of H2S from a gas stream with the properties of air, initially containing 2% H2S, so that the exit gas only contains 0.05% H2S. The H S-rich Selexol solvent is regenerated in a stripper using pure air. The absorber operates at 100 psig (6.8 atm gauge) and 70°F. The stripper operates at 1 atm absolute and 200°F. The stripper reduces the H S content of the Selexol solvent to 0.5%, which is the feed concentration to the absorber. The gas to be treated is flowing at 1000 kmol/h, and it may be assumed that the Selexol solvent circulates at 30,000 kg/h. In the packed-bed absorber, it is known that HoV = 3.5 m. In the packed-bed stripper, it is known that HoV = 0.25 m. It is proposed to use an existing stripper that is packed with a 5 m high section of 1.5-in, ceramic Raschig rings and has a diameter of 1.8 m.
1. Determine the required absorber height. You may assume that the Colburn method is valid.
2. Determine the exit H2S mole fraction in the Selexol solvent. Comment on this value.
3. For the stripper, determine the amount of air needed.
4. At what percentage of flooding is the stripper operating? Do you recommend operating under these conditions?
5. Determine the pressure drop across the stripper, in kPa.
Step by Step Answer:

Analysis Synthesis And Design Of Chemical Processes
ISBN: 9780134177403
5th Edition
Authors: Richard Turton, Joseph Shaeiwitz, Debangsu Bhattacharyya, Wallace Whiting





