Bovine pancreatic trypsin inhibitor (BPTI; Figure 6.25) contains six cysteine residues that form three disulfide bonds in
Question:
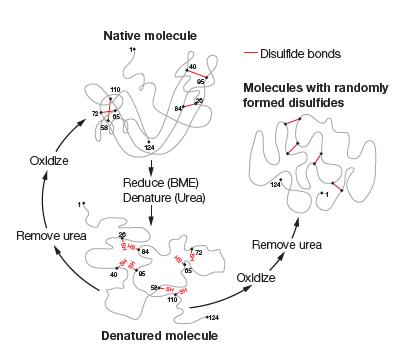
Bovine pancreatic trypsin inhibitor (BPTI; Figure 6.25) contains six cysteine residues that form three disulfide bonds in the native structure of BPTI. Suppose BPTI is reduced and unfolded in urea (as illustrated for RNase A in Figure 6.22). If the reduced unfolded protein were oxidized prior to the removal of the urea, what fraction of the resulting mixture would you expect to possess native disulfide bonds?
Figure 6.25
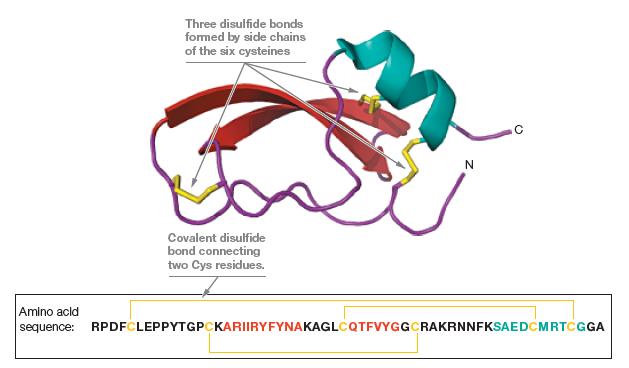
Figure 6.22
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Biochemistry Concepts And Connections
ISBN: 9780134641621
2nd Edition
Authors: Dean Appling, Spencer Anthony-Cahill, Christopher Mathews
Question Posted:





