Question: You need to make a buffer whose pH is 7.0, and you can choose from the weak acids shown in Table 2.6. Briefly explain your
You need to make a buffer whose pH is 7.0, and you can choose from the weak acids shown in Table 2.6. Briefly explain your choice.
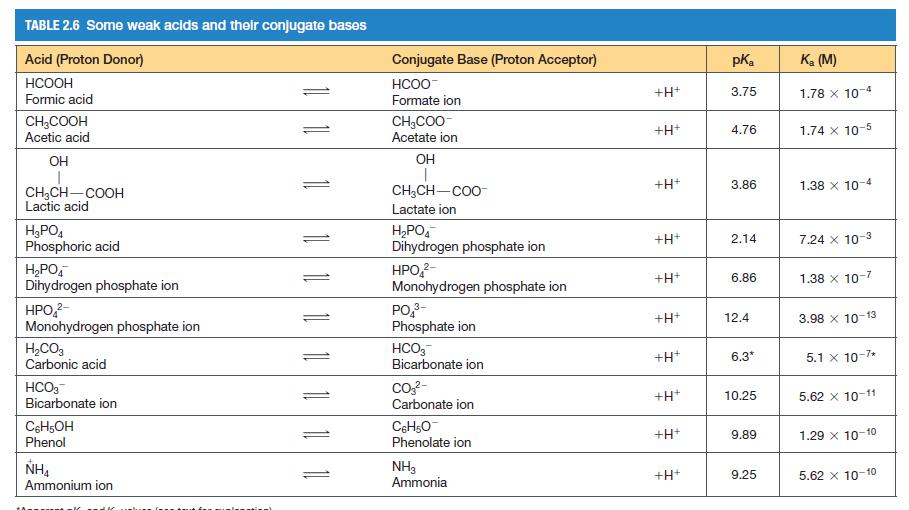
TABLE 2.6 Some weak acids and their conjugate bases Acid (Proton Donor) HCOOH Formic acid CH3COOH Acetic acid OH CHCH-COOH Lactic acid HPO4 Phosphoric acid HPO4 Dihydrogen phosphate ion HPO - Monohydrogen phosphate ion HCO3 Carbonic acid HCO3 Bicarbonate ion C6H5OH Phenol NHA Ammonium ion 11 11 11 1 Conjugate Base (Proton Acceptor) HCOO Formate ion CHCOO- Acetate ion OH T CHCH-COO Lactate ion HPO4 Dihydrogen phosphate ion HPO- Monohydrogen phosphate ion PO - Phosphate ion HCO3 Bicarbonate ion CO3- Carbonate ion C6H5O Phenolate ion NH3 Ammonia +H+ +H+ +H+ +H+ +H+ +H+ +H+ +H* +H+ +H+ pK 3.75 4.76 3.86 2.14 6.86 12.4 6.3* 10.25 9.89 9.25 K (M) 1.78 x 10-4 1.74 x 10-5 1.38 x 10-4 7.24 x 10-3 1.38 x 10-7 3.98 x 10-13 5.1 x 10-7* 5.62 X 10-11 1.29 X 10-10 5.62 x 10-10
Step by Step Solution
3.42 Rating (155 Votes )
There are 3 Steps involved in it
To create a buffer solution with a pH of 70 you should choose a weak acid and its conjugate base tha... View full answer

Get step-by-step solutions from verified subject matter experts


