The Ideal Gas Law states that PV = nRT, where P is pressure, Vis volume, n is
Question:
The Ideal Gas Law states that PV = nRT, where P is pressure, Vis volume, n is the number of moles of gas, R is a fixed constant (the gas constant), and T' is absolute temperature. Show that
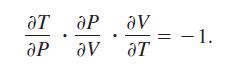
Transcribed Image Text:
aT ар ар Ꮩ . av aT - 1.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (6 reviews)
aT PV T V PV VT n RT xB PV n x...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Mathematics questions
-
The ideal gas law relates the pressure P, volume V, and temperature T of an ideal gas: PV = nRT where n is the number of moles R = 8.31145 Plots of pressure versus volume (four curves " in one plot)...
-
The ideal gas law relates the pressure P, volime V, and temperature T of an ideal gas: PV= nRT where " is the number of moles and R = 8.3145 J/(K. mol). Plots of pressure versus volume at constant...
-
The Ideal Gas Law is PV = nRT, where "n" is the number of moles and "R= 8.314 J/mol.K" is the Universal Gas Constant. 1. Define P, V, and T in the equation above and give the unit for each term in...
-
(1.71, 2.05) Use the confidence interval to find the margin of error and the sample mean.
-
Identify the animals most likely to carry rabies in the United States. Why is rabies so rare in humans and domesticated animals in developed countries?
-
What critical economic role does the financial system play in the economy?
-
Describe the importance of a multidisciplinary approach to patient care.
-
Surepar Disc Golf Course was opened on March 1 by Bill Arnsdorf. The following selected events and transactions occurred during March: Mar. 1 Invested $60,000 cash in the business in exchange for...
-
Garcia Company sells snowboards. Each snowboard requires direct materials of $110, direct labor of $40, variable overhead of $55, and variable selling, general, and administrative costs of $13. The...
-
A pharmaceutical corporation has two plants that produce the same over-the-counter medicine. If x 1 and x 2 are the numbers of units produced at plant 1 and plant 2, respectively, then the total...
-
The utility function U = (x, y) is a measure of the utility (or satisfaction) derived by a person from the consumption of two products x and y. The utility function for two products is (a) Determine...
-
In 2016, Deon and NeNe are married filing jointly. They have three dependent children under 18 years of age. Deon and NeNe's AGI is $811,300, their taxable income is $720,250, and they itemize their...
-
What unambiguous principal of finance, agreed to by virtually all economists, is violated by the accounting standards used by state and local governments to calculate how much their pension plans are...
-
Memory has 5 1 2 Mi ( mebi - the binary equivalent of mega ) cells. The cache is directly addressable. The tags are 1 2 bits. The lines have 8 cells of 3 2 bits. How many ki lines are there in the...
-
The auditor builds a model using inputs including the date of acquisition of each new long-lived asset acquired during the year, its acquisition cost, useful life, and salvage value. The auditor will...
-
Peter O Day , Manager, Technical and Communications needs you to set up a secure communications infrastructure for remote employees that may be working from home or on the road. What can be used to...
-
All traffic between each branch office should be encrypted automatically. Which method of encryption is best for LAN - LAN encryption? What is necessary to set this up ?
-
Greenfor is a large producer of lumber and paper products. The company has a periodic inventory system and uses the weighted-average cost flow assumption. You gathered the following information...
-
Derive Eq. (18.33) from Eq. (18.32).
-
The ________ of A with B consists of all elements in both A and B.
-
True or False. The intersection of two sets is always a subset of their union.
-
True or False. If A is a set, the complement of A is the set of all the elements in the universal set that are not in A.
-
January 1 2 0 2 4 Nath - Langstrom Services, Incorporated , a computer software training firm, leased several computers under a two - year operating lease agreement from ComputerWorld Leasing which...
-
What are the implications of cognitive psychology and behavioral economics on the design and implementation of Reports and Proposals, particularly regarding decision-making processes and behavioral...
-
A soccer player performs a penalty kick. From a stationary static position, they run towards the ball and kick it towards the goal. Explain with as much detail as possible which biomechanical...

Study smarter with the SolutionInn App


